Toward Elucidating Epigenetic and Metabolic Regulation of Stem Cell Lineage Plasticity in Skin Aging
- PMID: 35663405
- PMCID: PMC9160930
- DOI: 10.3389/fcell.2022.903904
Toward Elucidating Epigenetic and Metabolic Regulation of Stem Cell Lineage Plasticity in Skin Aging
Abstract
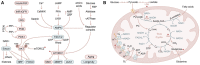
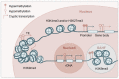
Skin is the largest organ in human body, harboring a plethora of cell types and serving as the organismal barrier. Skin aging such as wrinkling and hair graying is graphically pronounced, and the molecular mechanisms behind these phenotypic manifestations are beginning to unfold. As in many other organs and tissues, epigenetic and metabolic deregulations have emerged as key aging drivers. Particularly in the context of the skin epithelium, the epigenome and metabolome coordinately shape lineage plasticity and orchestrate stem cell function during aging. Our review discusses recent studies that proposed molecular mechanisms that drive the degeneration of hair follicles, a major appendage of the skin. By focusing on skin while comparing it to model organisms and adult stem cells of other tissues, we summarize literature on genotoxic stress, nutritional sensing, metabolic rewiring, mitochondrial activity, and epigenetic regulations of stem cell plasticity. Finally, we speculate about the rejuvenation potential of rate-limiting upstream signals during aging and the dominant role of the tissue microenvironment in dictating aged epithelial stem cell function.
Keywords: epigenetics; inflammaging; metabolism; skin aging; stem cell lineage plasticity.
Copyright © 2022 Lyu and Ge.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
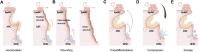

Similar articles
-
Regulation of Stem Cell Aging by Metabolism and Epigenetics.Cell Metab. 2017 Sep 5;26(3):460-474. doi: 10.1016/j.cmet.2017.07.019. Epub 2017 Aug 17. Cell Metab. 2017. PMID: 28826795 Review.
-
The circadian clock in skin: implications for adult stem cells, tissue regeneration, cancer, aging, and immunity.J Biol Rhythms. 2015 Jun;30(3):163-82. doi: 10.1177/0748730414563537. Epub 2015 Jan 13. J Biol Rhythms. 2015. PMID: 25589491 Free PMC article. Review.
-
Stem Cell Rejuvenation by Restoration of Youthful Metabolic Compartmentalization.Rejuvenation Res. 2021 Dec;24(6):470-474. doi: 10.1089/rej.2021.0076. Rejuvenation Res. 2021. PMID: 34846176
-
Deciphering the molecular mechanisms of stem cell dynamics in hair follicle regeneration.Exp Mol Med. 2024 Feb;56(1):110-117. doi: 10.1038/s12276-023-01151-5. Epub 2024 Jan 5. Exp Mol Med. 2024. PMID: 38182654 Free PMC article. Review.
-
Transcriptional and signalling regulation of skin epithelial stem cells in homeostasis, wounds and cancer.Exp Dermatol. 2021 Apr;30(4):529-545. doi: 10.1111/exd.14247. Epub 2020 Dec 11. Exp Dermatol. 2021. PMID: 33249665 Free PMC article. Review.
Cited by
-
Irradiation-induced hair graying in mice: an experimental model to evaluate the effectiveness of interventions targeting oxidative stress, DNA damage prevention, and cellular senescence.Geroscience. 2024 Jun;46(3):3105-3122. doi: 10.1007/s11357-023-01042-7. Epub 2024 Jan 6. Geroscience. 2024. PMID: 38182857 Free PMC article. Review.
-
Skin Cancer Microenvironment: What We Can Learn from Skin Aging?Int J Mol Sci. 2023 Sep 13;24(18):14043. doi: 10.3390/ijms241814043. Int J Mol Sci. 2023. PMID: 37762344 Free PMC article. Review.
-
Autophagy Dysfunction: The Kernel of Hair Loss?Clin Cosmet Investig Dermatol. 2024 May 20;17:1165-1181. doi: 10.2147/CCID.S462294. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 38800357 Free PMC article. Review.
-
Skin health and biological aging.Nat Aging. 2025 Jul;5(7):1195-1206. doi: 10.1038/s43587-025-00901-6. Epub 2025 Jun 17. Nat Aging. 2025. PMID: 40527938 Review.
-
Overview of chromatin regulatory processes during surface ectodermal development and homeostasis.Dev Biol. 2024 Nov;515:30-45. doi: 10.1016/j.ydbio.2024.07.001. Epub 2024 Jul 4. Dev Biol. 2024. PMID: 38971398 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

