Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion-Positive NSCLC
- PMID: 35663414
- PMCID: PMC9160474
- DOI: 10.1016/j.jtocrr.2022.100332
Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion-Positive NSCLC
Abstract
Introduction: Entrectinib is an approved tyrosine kinase inhibitor (TKI) for ROS1 fusion-positive NSCLC. An updated integrated analysis of entrectinib from the ALKA-372-001, STARTRK-1, and STARTRK-2 trials is presented, with substantially longer follow-up, more patients, and the first description of the median overall survival (OS). An exploratory analysis of entrectinib in ROS1 fusion-positive NSCLC with the central nervous system (CNS)-only progression post-crizotinib is reported.
Methods: Adults with ROS1 fusion-positive, locally advanced or metastatic NSCLC who received at least one dose of entrectinib and had 12 months or longer of follow-up were included in the analysis. Co-primary end points were confirmed objective response rate (ORR) and duration of response (DoR) by blinded independent central review. The data cutoff was on August 31, 2020.
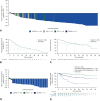
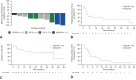
Results: The efficacy-assessable population comprised 168 ROS1 TKI-naïve patients. The median survival follow-up was 29.1 months (interquartile range, 21.8-35.9). The ORR was 68% (95% confidence interval [CI]: 60.2-74.8); the median DoR was 20.5 months. The median progression-free survival (PFS) was 15.7 months and the median OS was 47.8 months. In the 25 patients with measurable baseline CNS metastases, the intracranial ORR was 80% (95% CI: 59.3-93.2), median intracranial DoR was 12.9 months, and median intracranial PFS was 8.8 months. Among 18 patients with CNS-only progression on previous crizotinib treatment, two achieved a partial response (11%) and four had stable disease (22%). In seven patients with measurable CNS disease from this cohort, the intracranial ORR was 14% (1 partial response).
Conclusions: Entrectinib is active and achieves prolonged survival in ROS1 TKI-naïve patients with ROS1 fusion-positive NSCLC. Modest activity is seen in patients with CNS-only progression post-crizotinib.
Keywords: Entrectinib; Intracranial efficacy; NSCLC; ROS1 fusions; Treatment post-crizotinib.
© 2022 The Authors.
Figures