A simple method to align cells on 3D hydrogels using 3D printed molds
- PMID: 35663509
- PMCID: PMC9165732
- DOI: 10.1016/j.bea.2021.100001
A simple method to align cells on 3D hydrogels using 3D printed molds
Abstract
Vascular smooth muscle cells align circumferentially around the vessel lumen, which allows these cells to control vascular tone by contracting and relaxing. It is essential that this circumferential alignment is recapitulated in tissue engineered blood vessels. While many methods have been reported to align cells on 2D polymeric substrates, few techniques enable cell alignment on a 3D physiologically relevant hydrogel substrate. We hypothesized that the ridges inherent to the sides of fused deposition modeling 3D printed molds could be used to topographically pattern both stiff and soft substrates and thereby align cells on flat and curved surfaces. Flat and curved molds with 150, 250, and 350 μm ridges were 3D printed and used to topographically pattern polydimethylsiloxane and gelatin-methacryloyl. The ridges transferred to both substrates with less than 10% change in ridge size. Vascular smooth muscle cells were then seeded on each substrate, and nuclear and actin alignment were quantified. Cells were highly aligned with the molded ridges to a similar extent on both the stiffer polydimethylsiloxane and the softer gelatin-methacryloyl substrates. These data confirm that fused deposition modeling 3D printed molds are a rapid, cost-effective way to topographically pattern stiff and soft substrates in varied 3D shapes. This method will enable investigators to align cells on 3D polymeric and hydrogel structures for tissue engineering and other applications.
Keywords: 3D printing; Cell alignment; Hydrogel patterning; Vascular smooth muscle cells.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




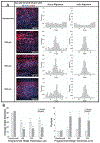
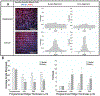
References
-
- Li Y, et al., Engineering cell alignment in vitro, Biotechnol. Adv 32 (2) (2014) 347–365. - PubMed
-
- Dartsch PC, Hammerle H, Orientation response of arterial smooth muscle cells to mechanical stimulation, Eur. J. Cell Biol 41 (2) (1986) 339–346. - PubMed
-
- Kemeny SF, et al., Glycated collagen alters endothelial cell actin alignment and nitric oxide release in response to fluid shear stress, J. Biomech 44 (10) (2011) 1927–1935. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
