Optimizing Foundational Therapies in Patients With HFrEF: How Do We Translate These Findings Into Clinical Care?
- PMID: 35663626
- PMCID: PMC9156437
- DOI: 10.1016/j.jacbts.2021.10.018
Optimizing Foundational Therapies in Patients With HFrEF: How Do We Translate These Findings Into Clinical Care?
Abstract
Given the high risk of adverse outcomes in patients with heart failure and reduced ejection fraction (HFrEF), there is an urgent need for the initiation and titration of guideline-directed medical therapy (GDMT) that can reduce the risk of morbidity and mortality. Clinical practice guidelines are now emphasizing the need for early and rapid initiation of therapies that have cardiovascular benefit. Recognizing that there are many barriers to GDMT initiation and optimization, health care providers should aim to introduce the 4 pillars of quadruple therapy now recommended by most clinical practice guidelines: angiotensin receptor-neprilysin inhibitors, beta-blockers, mineralocorticoid receptor antagonists, and sodium-glucose co-transporter 2 inhibitors. A large proportion of patients with HFrEF do not have clinical contraindications to GDMT but are not treated with these therapies. Early initiation of low-dose combination therapy should be tolerated by most patients. However, patient-related factors such as hemodynamics, frailty, and laboratory values will need consideration for maximum tolerated GDMT. GDMT initiation in acute heart failure hospitalization represents another important avenue to improve use of GDMT. Finally, removal of therapies that do not have clear cardiovascular benefit should be considered to lower polypharmacy and reduce the risk of adverse side effects. Future prospective studies aimed at guiding optimal implementation of quadruple therapy are warranted to reduce morbidity and mortality in patients with HFrEF.
Keywords: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta-blocker; GDMT, guideline-directed medical therapy; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor agonist; SGLT2i, sodium–glucose co-transporter 2 inhibitor; T2DM, type 2 diabetes mellitus; angiotensin receptor–neprilysin inhibitor; beta-blockers; eGFR, estimated glomerular filtration rate; mineralocorticoid receptor antagonists; sodium–glucose co-transporter 2 inhibitors.
© 2022 The Authors.
Conflict of interest statement
The funding support for the think tank meeting was provided through unrestricted grants from AstraZeneca Canada and Boehringer Ingelheim. Dr Sharma has received support from the Fonds de Recherche Santé Quebec (FRSQ) Junior 1 clinician scholars program, Canada Institute for Health Research (CIHR grant #175095), Roche Diagnostics, Boeringer Ingelheim, Novartis, and Takeda. Dr Verma holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and has received research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization. Dr Bhatt discloses the following relationships: advisory board, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, and Regado Biosciences; board of directors, Boston VA Research Institute, Society of Cardiovascular Patient Care, and ToBeSoft; Chair, Inaugural Chair, American Heart Association Quality Oversight Committee; data monitoring committees, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; honoraria, American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other, Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), and VA CART Research and Publications Committee (Chair); research funding, Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company, and 89Bio; royalties, Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator, Abbott, Biotronik, Boston Scientific, CSI, St Jude Medical (now Abbott), Philips, and Svelte; trustee, American College of Cardiology; and unfunded research, FlowCo, Merck, and Takeda. Dr Connelly has received research grants to his institution from AstraZeneca and Boehringer Ingelheim; received support for travel to scientific meeting from Boehringer Ingelheim and honoraria for speaking engagements and ad hoc participation in advisory boards from AstraZeneca, Boehringer Ingelheim, and Janssen. Dr Swiggum has received research grants from AstraZeneca, Boehringer Ingelheim, and Novartis; advisory or consulting honorarium from Akcea Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, the Boehringer Ingelheim–Lilly Alliance, Novartis, Pfizer, and Servier. Dr Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, and Relypsa; speaker engagements with Novartis and Roche Diagnostics; and participates on clinical endpoint committees for studies sponsored by Galmed and Novartis. Dr Zieroth has received research grant support or served on advisory boards for and speaking engagements with Abbott, Akcea, AstraZeneca, Amgen, Alnylam, Bayer, Boehringer Ingelheim, Eli-Lilly, Merck, Novartis, Otsuka, Pfizer, Servier, and Vifor; and serves on a clinical trial steering committee or as a National Lead for studies sponsored by AstraZeneca, Boehringer Ingelheim, and Novartis. Dr Butler is a consultant to Abbott, Adrenomed, Amgen, Array, AstraZeneca, Bayer, Berlin Cures, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sanofi, and Vifor.
Figures

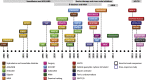


References
-
- Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. - PubMed
-
- Sharma A., Zhao X., Hammill B.G., et al. Trends in noncardiovascular comorbidities among patients hospitalized for heart failure. Circ Hear Fail. 2018;11(6) - PubMed
-
- Maddox T.M., Januzzi J.L., Allen L.A., et al. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810. - PubMed
-
- McDonald M., Virani S., Chan M., et al. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol. 2021;37(4):531–546. - PubMed
-
- Vaduganathan M., Claggett B.L., Jhund P.S., et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121–128. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

