Increased amphiregulin expression by CD4+ T cells from individuals with asymptomatic Leishmania donovani infection
- PMID: 35663920
- PMCID: PMC9136704
- DOI: 10.1002/cti2.1396
Increased amphiregulin expression by CD4+ T cells from individuals with asymptomatic Leishmania donovani infection
Abstract
Objectives: There is an urgent need to be able to identify individuals with asymptomatic Leishmania donovani infection, so their risk of progressing to VL and transmitting parasites can be managed. This study examined transcriptional markers expressed by CD4+ T cells that could distinguish asymptomatic individuals from endemic controls and visceral leishmaniasis (VL) patients.
Methods: CD4+ T cells were isolated from individuals with asymptomatic L. donovani infection, endemic controls and VL patients. RNA was extracted and RNAseq employed to identify differentially expressed genes. The expression of one gene and its protein product during asymptomatic infection were evaluated.
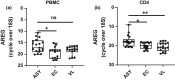
Results: Amphiregulin (AREG) was identified as a distinguishing gene product in CD4+ T cells from individuals with asymptomatic L. donovani infection, compared to VL patients and healthy endemic control individuals. AREG levels in plasma and antigen-stimulated whole-blood assay cell culture supernatants were significantly elevated in asymptomatic individuals, compared to endemic controls and VL patients. Regulatory T (Treg) cells were identified as an important source of AREG amongst CD4+ T-cell subsets in asymptomatic individuals.
Conclusion: Increased Treg cell AREG expression was identified in individuals with asymptomatic L. donovani infection, suggesting the presence of an ongoing inflammatory response in these individuals required for controlling infection and that AREG may play an important role in preventing inflammation-induced tissue damage and subsequent disease in asymptomatic individuals.
Keywords: CD4+ T cell; Leishmania donovani; amphiregulin; regulatory T cell; visceral leishmaniasis.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ismael A, Zijlstra EE, Ghalib HW, El‐Hassan AM. Endemic kala‐azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post‐kala‐azar dermal leishmaniasis. Am J Trop Med Hyg 1994; 51: 826–836. - PubMed
-
- Schaefer KU, Kurtzhals JA, Gachihi GS, Muller AS, Kager PA. A prospective sero‐epidemiological study of visceral leishmaniasis in Baringo District, Rift Valley Province, Kenya. Trans R Soc Trop Med Hyg 1995; 89: 471–475. - PubMed
-
- Ali A, Ashford RW. Visceral leishmaniasis in Ethiopia. IV. Prevalence, incidence and relation of infection to disease in an endemic area. Ann Trop Med Parasitol 1994; 88: 289–293. - PubMed
-
- Evans TG, Teixeira MJ, McAuliffe IT et al. Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis 1992; 166: 1124–1132. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
