The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis
- PMID: 35663975
- PMCID: PMC9161083
- DOI: 10.3389/fimmu.2022.867260
The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis
Abstract
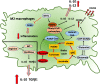
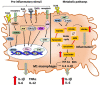
Innate and adaptive immunity represent a harmonic counterbalanced system involved in the induction, progression, and possibly resolution of the inflammatory reaction that characterize autoimmune rheumatic diseases (ARDs), including rheumatoid arthritis (RA). Although the immunopathophysiological mechanisms of the ARDs are not fully clarified, they are often associated with an inappropriate macrophage/T-cell interaction, where classical (M1) or alternative (M2) macrophage activation may influence the occurrence of T-helper (Th)1 or Th2 responses. In RA patients, M1/Th1 activation occurs in an inflammatory environment dominated by Toll-like receptor (TLR) and interferon (IFN) signaling, and it promotes a massive production of pro-inflammatory cytokines [i.e., tumor necrosis factor-α (TNFα), interleukin (IL)-1, IL-12, IL-18, and IFNγ], chemotactic factors, and matrix metalloproteinases resulting in osteoclastogenesis, erosion, and progressive joint destruction. On the other hand, the activation of M2/Th2 response determines the release of growth factors and cytokines [i.e., IL-4, IL-10, IL-13, and transforming growth factor (TGF)-β] involved in the anti-inflammatory process leading to the clinical remission of RA. Several subtypes of macrophages have been described. Five polarization states from M1 to M2 have been confirmed in in vitro studies analyzing morphological characteristics, gene expression of phenotype markers (CD80, CD86, TLR2, TLR4, or CD206, CD204, CD163, MerTK), and functional aspect, including the production of reactive oxygen species (ROS). An M1 and M2 macrophage imbalance may induce pathological consequences and contribute to several diseases, such as asthma or osteoclastogenesis in RA patients. In addition, the macrophage dynamic polarization from M1 to M2 includes the presence of intermediate polarity stages distinguished by the expression of specific surface markers and the production/release of distinct molecules (i.e., nitric oxide, cytokines), which characterize their morphological and functional state. This suggests a "continuum" of macrophage activation states playing an important role during inflammation and its resolution. This review discusses the importance of the delicate M1/M2 imbalance in the different phases of the inflammatory process together with the identification of specific pathways, cytokines, and chemokines involved, and its clinical outcomes in RA. The analysis of these aspects could shed a light on the abnormal inflammatory activation, leading to novel therapeutical approaches which may contribute to restore the M1/M2 balance.
Keywords: Inflammation; Macrophage polarization; Rheumatoid anhritis; Synovitis; bDMARD therapy.
Copyright © 2022 Cutolo, Campitiello, Gotelli and Soldano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

