Mechanisms, Challenges, and Opportunities of Dual Ni/Photoredox-Catalyzed C(sp2)-C(sp3) Cross-Couplings
- PMID: 35664524
- PMCID: PMC9162266
- DOI: 10.1002/wcms.1573
Mechanisms, Challenges, and Opportunities of Dual Ni/Photoredox-Catalyzed C(sp2)-C(sp3) Cross-Couplings
Abstract
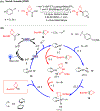
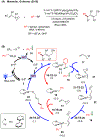
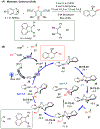
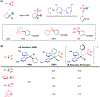
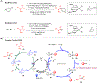
The merging of photoredox and nickel catalysis has revolutionized the field of C-C cross-coupling. However, in comparison to the development of synthetic methods, detailed mechanistic investigations of these catalytic systems are lagging. To improve the mechanistic understanding, computational tools have emerged as powerful tools to elucidate the factors controlling reactivity and selectivity in these complex catalytic transformations. Based on the reported computational studies, it appears that the mechanistic picture of catalytic systems is not generally applicable, but is rather dependent on the specific choice of substrate, ligands, photocatalysts, etc. Given the complexity of these systems, the need for more accurate computational methods, readily available and user-friendly dynamics simulation tools, and data-driven approaches is clear in order to understand at the molecular level the mechanisms of these transformations. In particular, we anticipate that such improvement of theoretical methods will become crucial to advance the understanding of excited-state properties and dynamics of key species, as well as to enable faster and unbiased exploration of reaction pathways. Further, with greater collaboration between computational, experimental, and spectroscopic communities, the mechanistic investigation of photoredox/Ni dual-catalytic reactions is expected to thrive quickly, facilitating the design of novel catalytic systems and promoting our understanding of the reaction selectivity.
Keywords: C-C cross-coupling; Photoredox/nickel dual catalysis; density functional theory; metallaphotoredox catalysis; molecular dynamics; olefin difunctionalization; reaction selectivity.
Figures
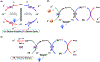





Similar articles
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
Dual Nickel- and Photoredox-Catalyzed Asymmetric Reductive Cross-Couplings: Just a Change of the Reduction System?Acc Chem Res. 2024 Jul 16;57(14):1997-2011. doi: 10.1021/acs.accounts.4c00309. Epub 2024 Jul 3. Acc Chem Res. 2024. PMID: 38961540
-
Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp(3)-sp(2) Cross-Coupling.Acc Chem Res. 2016 Jul 19;49(7):1429-39. doi: 10.1021/acs.accounts.6b00214. Epub 2016 Jul 5. Acc Chem Res. 2016. PMID: 27379472 Free PMC article.
-
Photoredox catalysis in nickel-catalyzed C-H functionalization.Beilstein J Org Chem. 2021 Aug 31;17:2209-2259. doi: 10.3762/bjoc.17.143. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34621388 Free PMC article. Review.
-
Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development.ACS Catal. 2017 Apr 7;7(4):2563-2575. doi: 10.1021/acscatal.7b00094. Epub 2017 Mar 14. ACS Catal. 2017. PMID: 28413692 Free PMC article. Review.
Cited by
-
Nickel-Catalyzed Radical Mechanisms: Informing Cross-Coupling for Synthesizing Non-Canonical Biomolecules.Acc Chem Res. 2023 Dec 19;56(24):3640-3653. doi: 10.1021/acs.accounts.3c00588. Epub 2023 Nov 30. Acc Chem Res. 2023. PMID: 38033206 Free PMC article.
-
Kinetics of a Ni/Ir-Photocatalyzed Coupling of ArBr with RBr: Intermediacy of ArNiII(L)Br and Rate/Selectivity Factors.J Am Chem Soc. 2022 Aug 24;144(33):15372-15382. doi: 10.1021/jacs.2c06831. Epub 2022 Aug 15. J Am Chem Soc. 2022. PMID: 35969479 Free PMC article.
-
Mechanisms of Photoredox Catalysis Featuring Nickel-Bipyridine Complexes.ACS Catal. 2024 May 29;14(11):9055-9076. doi: 10.1021/acscatal.4c02036. eCollection 2024 Jun 7. ACS Catal. 2024. PMID: 38868098 Free PMC article. Review.
-
Dual Nickel/Photoredox-Catalyzed Site-Selective Cross-Coupling of 1,2-Bis-Boronic Esters Enabled by 1,2-Boron Shifts.Angew Chem Int Ed Engl. 2022 Aug 22;61(34):e202207988. doi: 10.1002/anie.202207988. Epub 2022 Jul 14. Angew Chem Int Ed Engl. 2022. PMID: 35779000 Free PMC article.
-
Mechanism of Ni-Catalyzed Photochemical Halogen Atom-Mediated C(sp3)-H Arylation.J Am Chem Soc. 2024 Jun 5;146(22):15331-15344. doi: 10.1021/jacs.4c03099. Epub 2024 May 22. J Am Chem Soc. 2024. PMID: 38778454 Free PMC article.
References
-
- Twilton J, Zhang P, Shaw MH, Evans RW, MacMillan DW. The merger of transition metal and photocatalysis. Nat Rev Chem. 2017;1(7):1–19. 10.1038/s41570-017-0052 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
