Skeletal Stem Cells-A Paradigm Shift in the Field of Craniofacial Bone Tissue Engineering
- PMID: 35664558
- PMCID: PMC9161996
- DOI: 10.3389/fdmed.2020.596706
Skeletal Stem Cells-A Paradigm Shift in the Field of Craniofacial Bone Tissue Engineering
Abstract
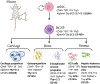
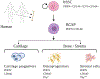
Defects of the craniofacial skeleton arise as a direct result of trauma, diseases, oncological resection, or congenital anomalies. Current treatment options are limited, highlighting the importance for developing new strategies to restore form, function, and aesthetics of missing or damaged bone in the face and the cranium. For optimal reconstruction, the goal is to replace "like with like." With the inherent challenges of existing options, there is a clear need to develop alternative strategies to reconstruct the craniofacial skeleton. The success of mesenchymal stem cell-based approaches has been hampered by high heterogeneity of transplanted cell populations with inconsistent preclinical and clinical trial outcomes. Here, we discuss the novel characterization and isolation of mouse skeletal stem cell (SSC) populations and their response to injury, systemic disease, and how their re-activation in vivo can contribute to tissue regeneration. These studies led to the characterization of human SSCs which are able to self-renew, give rise to increasingly fate restricted progenitors, and differentiate into bone, cartilage, and bone marrow stroma, all on the clonal level in vivo without prior in vitro culture. SSCs hold great potential for implementation in craniofacial bone tissue engineering and regenerative medicine. As we begin to better understand the diversity and the nature of skeletal stem and progenitor cells, there is a tangible future whereby a subset of human adult SSCs can be readily purified from bone or activated in situ with broad potential applications in craniofacial tissue engineering.
Keywords: craniofacial surgery; osteoprogenitors; skeletal progenitors; skeletal stem cells; tissue regeneration.
Conflict of interest statement
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Tissue Engineering and Regenerative Medicine in Craniofacial Reconstruction and Facial Aesthetics.J Craniofac Surg. 2020 Jan/Feb;31(1):15-27. doi: 10.1097/SCS.0000000000005840. J Craniofac Surg. 2020. PMID: 31369496 Free PMC article. Review.
-
Treatment of osteochondral defects in the rabbit's knee joint by implantation of allogeneic mesenchymal stem cells in fibrin clots.J Vis Exp. 2013 May 21;(75):e4423. doi: 10.3791/4423. J Vis Exp. 2013. PMID: 23728213 Free PMC article.
-
Skeletal stem and progenitor cells in bone development and repair.J Bone Miner Res. 2024 Jul 23;39(6):633-654. doi: 10.1093/jbmr/zjae069. J Bone Miner Res. 2024. PMID: 38696703 Review.
-
Skeletal Stem Cells: Origins, Functions and Uncertainties.Curr Mol Biol Rep. 2017 Dec;3(4):236-246. doi: 10.1007/s40610-017-0075-5. Epub 2017 Oct 19. Curr Mol Biol Rep. 2017. PMID: 29430387 Free PMC article.
-
Stem Cells Regenerating the Craniofacial Skeleton: Current State-Of-The-Art and Future Directions.J Clin Med. 2020 Oct 15;9(10):3307. doi: 10.3390/jcm9103307. J Clin Med. 2020. PMID: 33076266 Free PMC article. Review.
Cited by
-
Revolutionizing bone regeneration: advanced biomaterials for healing compromised bone defects.Front Aging. 2023 Jul 14;4:1217054. doi: 10.3389/fragi.2023.1217054. eCollection 2023. Front Aging. 2023. PMID: 37520216 Free PMC article. Review.
-
A Biofabrication Strategy for a Custom-Shaped, Non-Synthetic Bone Graft Precursor with a Prevascularized Tissue Shell.Front Bioeng Biotechnol. 2022 Mar 9;10:838415. doi: 10.3389/fbioe.2022.838415. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35356783 Free PMC article.
References
-
- ASPS. Plastic Surgery Statistics American Society of Plastic and Reconstructive Surgeons. Arlington Heights, IL: American Society of Plastic Surgeons; (2019).
-
- Research PM. Bone Grafts and Substitutes Market Size, Share & Trends Analysis Report By Material Type (Natural, Synthetic); By Application Type (Spinal Fusion, Craniomaxillofacial, Long Bone); By Region-Segment Forecast, 2020–2026. San Francisco, CA: Grand View Research, Inc. (2020).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
