Could We Safely Avoid a Second Resection in Selected Patients With T1 Non-Muscle-Invasive Bladder Cancer? Preliminary Results of Cost-Effectiveness Study From HUmanitas New Indications for ReTUR (HuNIRe) Multicenter Prospective Trial
- PMID: 35664755
- PMCID: PMC9157494
- DOI: 10.3389/fonc.2022.879399
Could We Safely Avoid a Second Resection in Selected Patients With T1 Non-Muscle-Invasive Bladder Cancer? Preliminary Results of Cost-Effectiveness Study From HUmanitas New Indications for ReTUR (HuNIRe) Multicenter Prospective Trial
Abstract
Objectives: The aim of this study is to assess whether restaging transurethral resection (ReTUR) could be safely replaced with urine cytology (UC) and in-office fiexible cystoscopy in selected T1 non-muscle-invasive bladder cancer (NMIBC).
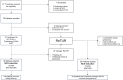
Materials and methods: This is an ongoing prospective multicenter trial enrolling patients diagnosed with T1 BC from 5 Italian centers. Patients with a macroscopically incomplete initial resection or absence of detrusor muscle were subjected to ReTUR according to European Association of Urology (EAU) guidelines. Conversely, those with a complete tumor resection at initial TUR underwent UC at 3-4 weeks and in-office fiexible white-light and narrow-band cystoscopy at 4-6 weeks. In case of positive UC, or evidence of recurrence at cystoscopy, ReTUR was performed within 2 weeks. Otherwise, patients started Bacillus Calmette-Guérin (BCG) induction course without ReTUR. The primary endpoint was to determine the feasibility and the clinical utility of not performing ReTUR in selected T1 NMIBC patients. The secondary endpoint was to perform a cost-benefit analysis of this alternative approach.
Results: Since May 2020, among 87 patients presenting with T1, 76 patients were enrolled. Nineteen (25%) patients underwent standard ReTUR after initial resection, 10 (13.2%) due to the absence of the detrusor muscle and 9 (11.8%) due to a macroscopically incomplete initial TUR. Overall, 57 (75%) patients initially avoided immediate ReTUR and underwent UC plus in-office flexible cystoscopy. Among them, 38 (66.7%) had no evidence of residual disease and immediately started the BCG induction course. Nineteen patients (33.3%) underwent "salvage" ReTUR due to either positive UC (7; 12.3%) or suspicious cystoscopy (12; 21%). Considering only the patients who initially avoided the ReTUR, disease recurrence was observed in 10/57. The saving of resource for each safely avoided ReTUR was estimated to be 1,759 €. Considering the entire sample, we estimated a saving of 855 € per patient if compared with the EAU guideline approach.
Conclusion: The preliminary results of our trial suggested that ReTUR might be safely avoided in highly selected T1 BC patients with a complete resection at first TUR. Longer follow-up and larger sample size are needed to investigate the long-term oncological outcomes of this alternative approach.
Keywords: cystoscopy; non-muscle-invasive bladder cancer; outcome; second resection; urine cytology.
Copyright © 2022 Contieri, Lughezzani, Buffi, Taverna, Giacobbe, Micheli, Barra, Colombo, Vanni, Guazzoni, Lazzeri, Hurle and HuNIRe Study Group.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Divrik RT, Sahin AF, Yildirim U, Altok M, Zorlu F. Impact of Routine Second Transurethral Resection on the Long-Term Outcome of Patients With Newly Diagnosed Pt1 Urothelial Carcinoma With Respect to Recurrence, Progression Rate, and Disease-Specific Survival: A Prospective Randomised Clinical Trial. Eur Urol (2010) 58:185–90. doi: 10.1016/j.eururo.2010.03.007 - DOI - PubMed
-
- Krajewski W, Nowak Ł, Poletajew S, Tukiendorf A, Moschini M, Mari A, et al. . The Impact of Restaging Transurethral Resection of Bladder Tumor on Survival Parameters in T1 Nonmuscle-Invasive Bladder Cancer: Systematic Review and Meta-Analysis. J Endourol (2020) 34(8):795–804. doi: 10.1089/end.2020.0301 - DOI - PubMed
-
- Gontero P, Sylvester R, Pisano F, Joniau S, Oderda M, Serretta V, et al. . The Impact of Re-Transurethral Resection on Clinical Outcomes in a Large Multicentre Cohort of Patients With T1 High-Grade/Grade 3 Bladder Cancer Treated With Bacille Calmette-Guérin. BJU Int (2016) 118(1):44–52. doi: 10.1111/bju.13354 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources