Clinical Course and Diagnostic Findings of Biopsy Controlled Presumed Immune-Mediated Polyneuropathy in 70 European Cats
- PMID: 35664840
- PMCID: PMC9156799
- DOI: 10.3389/fvets.2022.875657
Clinical Course and Diagnostic Findings of Biopsy Controlled Presumed Immune-Mediated Polyneuropathy in 70 European Cats
Abstract
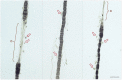
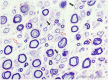
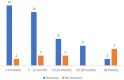
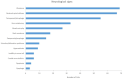
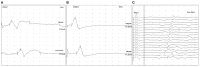
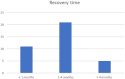
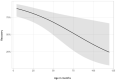
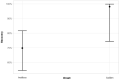
There is a paucity of information on the clinical course and outcome of young cats with polyneuropathy. The aim of the study was to describe the clinical features, diagnostic investigations, and outcome of a large cohort of cats with inflammatory polyneuropathy from several European countries. Seventy cats with inflammatory infiltrates in intramuscular nerves and/or peripheral nerve biopsies were retrospectively included. Information from medical records and follow up were acquired via questionnaires filled by veterinary neurologists who had submitted muscle and nerve biopsies (2011-2019). Median age at onset was 10 months (range: 4-120 months). The most common breed was British short hair (25.7%), followed by Domestic short hair (24.3%), Bengal cat (11.4%), Maine Coon (8.6%) and Persian cat (5.7%), and 14 other breeds. Male cats were predominantly affected (64.3%). Clinical signs were weakness (98.6%) and tetraparesis (75.7%) in association with decreased withdrawal reflexes (83.6%) and, less commonly, cranial nerve signs (17.1%), spinal pain/hyperesthesia (12.9%), and micturition/defecation problems (14.3%). Onset was sudden (30.1%) or insidious (69.1%), and an initial progressive phase was reported in 74.3%. Characteristic findings on electrodiagnostic examination were presence of generalized spontaneous electric muscle activity (89.6%), decreased motor nerve conduction velocity (52.3%), abnormal F-wave studies (72.4%), pattern of temporal dispersion (26.1%) and unremarkable sensory tests. The clinical course was mainly described as remittent (49.2%) or remittent-relapsing (34.9%), while stagnation, progressive course or waxing and waning were less frequently reported. Relapses were common and occurred in 35.7% of the cats' population. An overall favorable outcome was reported in 79.4% of patients. In conclusion, young age at the time of diagnosis and sudden onset of clinical signs were significantly associated with recovery (p < 0.05). Clinical and electrodiagnostic features and the remittent-relapsing clinical course resembles juvenile chronic inflammatory demyelinating polyneuropathy (CIDP), as seen in human (children/adolescents), in many aspects.
Keywords: CIDP; GBS; electrodiagnostic; feline; neuromuscular; tetraparesis; weakness.
Copyright © 2022 van Renen, Fischer, Kolb, Wielaender, Zablotski, Nessler, Tipold, Cappello, Flegel, Loderstedt, Gnirs, Rentmeister, Rupp, von Klopmann, Steffen, Jurina, Del Vecchio, Deutschland, König, Gandini, Harcourt-Brown, Kornberg, Bianchi, Gagliardo, Menchetti, Schenk, Tabanez, Matiasek and Rosati.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

