Engineering Shewanella carassii, a newly isolated exoelectrogen from activated sludge, to enhance methyl orange degradation and bioelectricity harvest
- PMID: 35664929
- PMCID: PMC9149024
- DOI: 10.1016/j.synbio.2022.04.010
Engineering Shewanella carassii, a newly isolated exoelectrogen from activated sludge, to enhance methyl orange degradation and bioelectricity harvest
Abstract
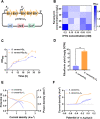
Electroactive microorganisms (EAMs) play important roles in biogeochemical redox processes and have been of great interest in the fields of energy recovery, waste treatment, and environmental remediation. However, the currently identified EAMs are difficult to be widely used in complex and diverse environments, due to the existence of poor electron transfer capability, weak environmental adaptability, and difficulty with engineering modifications, etc. Therefore, rapid and efficient screening of high performance EAMs from environments is an effective strategy to facilitate applications of microbial fuel cells (MFCs). In this study, to achieve efficient degradation of methyl orange (MO) by MFC and electricity harvest, a more efficient exoelectrogen Shewanella carassii-D5 that belongs to Shewanella spp. was first isolated from activated sludge by WO3 nanocluster probe technique. Physiological properties experiments confirmed that S. carassii-D5 is a Gram-negative strain with rounded colonies and smooth, slightly reddish surface, which could survive in media containing lactate at 30 °C. Moreover, we found that S. carassii-D5 exhibited remarkable MO degradation ability, which could degrade 66% of MO within 72 h, 1.7 times higher than that of Shewanella oneidensis MR-1. Electrochemical measurements showed that MFCs inoculated with S. carassii-D5 could generate a maximum power density of 704.6 mW/m2, which was 5.6 times higher than that of S. oneidensis MR-1. Further investigation of the extracellular electron transfer (EET) mechanism found that S. carassii-D5 strain had high level of c-type cytochromes and strong biofilm formation ability compared with S. oneidensis MR-1, thus facilitating direct EET. Therefore, to enhance indirect electron transfer and MO degradation capacity, a synthetic gene cluster ribADEHC encoding riboflavin synthesis pathway from Bacillus subtilis was heterologously expressed in S. carassii-D5, increasing riboflavin yield from 1.9 to 9.0 mg/g DCW with 1286.3 mW/m2 power density output in lactate fed-MFCs. Furthermore, results showed that the high EET rate endowed a faster degradation efficient of MO from 66% to 86% with a maximum power density of 192.3 mW/m2, which was 1.3 and 1.6 times higher than that of S. carassii-D5, respectively. Our research suggests that screening and engineering high-efficient EAMs from sludge is a feasible strategy in treating organic pollutants.
Keywords: Methyl orange; Microbial fuel cells; Riboflavin; Shewanella carassii; WO3 nanocluster probe.
© 2022 The Authors.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures





References
-
- Gude V.G. Wastewater treatment in microbial fuel cells–an overview. J Clean Prod. 2016;122(20):287–307. doi: 10.1016/j.jclepro.2016.02.022. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

