This is a preprint.
SARS-CoV-2 diagnostic testing rates determine the sensitivity of genomic surveillance programs
- PMID: 35664998
- PMCID: PMC9164450
- DOI: 10.1101/2022.05.20.22275319
SARS-CoV-2 diagnostic testing rates determine the sensitivity of genomic surveillance programs
Update in
-
SARS-CoV-2 diagnostic testing rates determine the sensitivity of genomic surveillance programs.Nat Genet. 2023 Jan;55(1):26-33. doi: 10.1038/s41588-022-01267-w. Epub 2023 Jan 9. Nat Genet. 2023. PMID: 36624344 Free PMC article.
Abstract
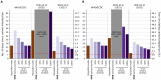
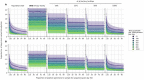
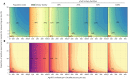
The first step in SARS-CoV-2 genomic surveillance is testing to identify infected people. However, global testing rates are falling as we emerge from the acute health emergency and remain low in many low- and middle-income countries (LMICs) (mean = 27 tests/100,000 people/day). We simulated COVID-19 epidemics in a prototypical LMIC to investigate how testing rates, sampling strategies, and sequencing proportions jointly impact surveillance outcomes and showed that low testing rates and spatiotemporal biases delay time-to-detection of new variants by weeks-to-months and can lead to unreliable estimates of variant prevalence even when the proportion of samples sequenced is increased. Accordingly, investments in wider access to diagnostics to support testing rates of ~100 tests/100,000 people/day could enable more timely detection of new variants and reliable estimates of variant prevalence. The performance of global SARS-CoV-2 genomic surveillance programs is fundamentally limited by access to diagnostic testing.
Conflict of interest statement
Declaration of interests: A.T., E.H., S.C., B.R. and B.E.N. declare that they are employed by FIND, the global alliance for diagnostics. J.A.S., M.D.P., S.B., M.V.K: The findings and conclusions in this manuscript are those of the authors and do not represent the official position of the World Health Organization.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
