The Ubiquitin Proteasome System and Nutrient Stress Response
- PMID: 35665152
- PMCID: PMC9161090
- DOI: 10.3389/fpls.2022.867419
The Ubiquitin Proteasome System and Nutrient Stress Response
Abstract
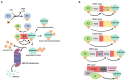
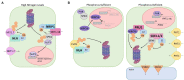
Plants utilize different molecular mechanisms, including the Ubiquitin Proteasome System (UPS) that facilitates changes to the proteome, to mitigate the impact of abiotic stresses on growth and development. The UPS encompasses the ubiquitination of selected substrates followed by the proteasomal degradation of the modified proteins. Ubiquitin ligases, or E3s, are central to the UPS as they govern specificity and facilitate the attachment of one or more ubiquitin molecules to the substrate protein. From recent studies, the UPS has emerged as an important regulator of the uptake and translocation of essential macronutrients and micronutrients. In this review, we discuss select E3s that are involved in regulating nutrient uptake and responses to stress conditions, including limited or excess levels of nitrogen, phosphorus, iron, and copper.
Keywords: 26S proteasome; abiotic stress; nutrient stress; nutrient uptake; protein degradation; ubiquitin ligase; ubiquitination.
Copyright © 2022 Mackinnon and Stone.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ahanger M. A., Ahmad P. (2019). Role of mineral nutrients in abiotic stress tolerance: revisiting the associated signaling mechanisms. Plant Signal. Mol, 269–285. doi: 10.1016/B978-0-12-816451-8.00016-2 - DOI
-
- Ayadi A., David P., Arrighi J.-F., Chiarenza S., Thibaud M.-C., Nussaume L., et al. . (2015). Reducing the genetic redundancy of Arabidopsis PHOSPHATE TRANSPORTER1 transporters to study phosphate uptake and signaling. Plant Physiol. 167, 1511–1526. doi: 10.1104/pp.114.252338, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

