High-Resolution 3D Heart Models of Cardiomyocyte Subpopulations in Cleared Murine Heart
- PMID: 35665220
- PMCID: PMC9158482
- DOI: 10.3389/fphys.2022.779514
High-Resolution 3D Heart Models of Cardiomyocyte Subpopulations in Cleared Murine Heart
Abstract
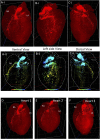
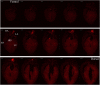
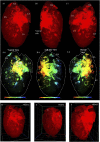
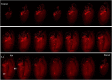
Biological tissues are naturally three-dimensional (3D) opaque structures, which poses a major challenge for the deep imaging of spatial distribution and localization of specific cell types in organs in biomedical research. Here we present a 3D heart imaging reconstruction approach by combining an improved heart tissue-clearing technique with high-resolution light-sheet fluorescence microscopy (LSFM). We have conducted a three-dimensional and multi-scale volumetric imaging of the ultra-thin planes of murine hearts for up to 2,000 images per heart in x-, y-, and z three directions. High-resolution 3D volume heart models were constructed in real-time by the Zeiss Zen program. By using such an approach, we investigated detailed three-dimensional spatial distributions of two specific cardiomyocyte populations including HCN4 expressing pacemaker cells and Pnmt+ cell-derived cardiomyocytes by using reporter mouse lines Hcn4DreER/tdTomato and PnmtCre/ChR2-tdTomato. HCN4 is distributed throughout right atrial nodal regions (i.e., sinoatrial and atrioventricular nodes) and the superior-inferior vena cava axis, while Pnmt+ cell-derived cardiomyocytes show distinct ventral, left heart, and dorsal side distribution pattern. Our further electrophysiological analysis indicates that Pnmt + cell-derived cardiomyocytes rich left ventricular (LV) base is more susceptible to ventricular arrhythmia under adrenergic stress than left ventricular apex or right ventricle regions. Thus, our 3D heart imaging reconstruction approach provides a new solution for studying the geometrical, topological, and physiological characteristics of specific cell types in organs.
Keywords: 3D volume heart models; HCN4 expression (HCN4+) pacemaker cells; Pnmt+ cell-derived cardiomyocytes (PdCMs); heart tissue-clearing; light-sheet fluorescence microscopy; optogenetics.
Copyright © 2022 Ren, Pu, Sun, Chen, Liu, Liu, O’Shea, Pavlovic, Tan and Lei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

