Central Commands to the Elbow and Shoulder Muscles During Circular Planar Movements of Hand With Simultaneous Generation of Tangential Forces
- PMID: 35665229
- PMCID: PMC9160871
- DOI: 10.3389/fphys.2022.864404
Central Commands to the Elbow and Shoulder Muscles During Circular Planar Movements of Hand With Simultaneous Generation of Tangential Forces
Abstract
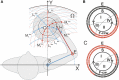
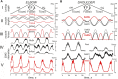
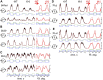
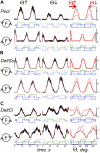
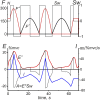
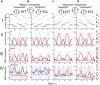
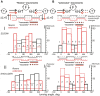
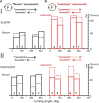
This study examines some of the non-linear effects of signal transduction in the human motor system, with particular emphasis on muscle hysteresis. The movement tests were analyzed in a group of eight subjects, which were asked to develop tangential force using visual biofeedback while performing slow, externally imposed, circular movements of right hand holding a moving handle operated by a computerized mechatronic system. The positional changes in the averaged EMGs of the elbow and shoulder muscles were compared for all combinations of direction of movement and generated force. Additionally, for one of the subjects, there was carried out MRI identification and 3D printing of the bones of the forelimb, shoulder, scapula and collarbone, which made it possible to reconstruct for him the length and force traces of all the muscles under study. The averaged EMG traces in muscles of both joints show their close correspondence to the related force traces, however, the co-activation patterns of activity in agonists and antagonists were also often encountered. The EMG waves related to the respective force waves were strongly dependent on the predominant direction of the muscle length changes within the correspondent force wave locations: the EMG intensities were higher for the shortening muscle movements (concentric contractions) and lower during muscle lengthening (eccentric contractions). The data obtained allows to suggest that for two-joint movements of the forelimbs, it is sufficient to consider the force and activation synergies (patterns of simultaneous activity in different muscles), ignoring at the first stage the effects associated with kinematic synergy. On the other hand, the data obtained indicate that the movement kinematics has a strong modulating effect on the activation synergy, dividing it into concentric and eccentric subtypes, in accordance with the known non-linear features of the muscle dynamics. It has been shown that the concentric and eccentric differences in the responses of the shoulder muscles are more clearly distinguishable than those in the elbow muscles. The shoulder muscles also have a more pronounced symmetry of the averaged EMG responses with respect to the ascending and descending phases of force waves, while demonstrating a lower degree of antagonist cocontraction. The data obtained suggest that the central commands in two-joint movements are determined mainly by the interdependence of force and activation synergies including both intra- and inter-joint components, while kinematic synergy can be interpreted as a potent modulator of activation synergy.
Keywords: electromyogram; forelimb; motor commands; motor control; muscle synergy; two-joint movements.
Copyright © 2022 Kostyukov, Gorkovenko, Kulyk, Lehedza, Shushuiev, Zasada and Strafun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Movement- and Load-Dependent Differences in the EMG Patterns of the Human Arm Muscles during Two-Joint Movements (A Preliminary Study).Front Physiol. 2016 Jun 8;7:218. doi: 10.3389/fphys.2016.00218. eCollection 2016. Front Physiol. 2016. PMID: 27375496 Free PMC article.
-
Hysteresis and Synergy of the Central Commands to Muscles Participating in Parafrontal Upper Limb Movements.Front Physiol. 2019 Nov 26;10:1441. doi: 10.3389/fphys.2019.01441. eCollection 2019. Front Physiol. 2019. PMID: 31849699 Free PMC article.
-
The Averaged EMGs Recorded from the Arm Muscles During Bimanual "Rowing" Movements.Front Physiol. 2015 Nov 27;6:349. doi: 10.3389/fphys.2015.00349. eCollection 2015. Front Physiol. 2015. PMID: 26640440 Free PMC article.
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
-
The control and perception of antagonist muscle action.Exp Brain Res. 2023 Jan;241(1):1-12. doi: 10.1007/s00221-022-06498-1. Epub 2022 Oct 30. Exp Brain Res. 2023. PMID: 36309879 Review.
Cited by
-
Hysteresis reveals a happiness bias effect in dynamic emotion recognition from ambiguous biological motion.J Vis. 2023 Nov 1;23(13):5. doi: 10.1167/jov.23.13.5. J Vis. 2023. PMID: 37962533 Free PMC article.
References
-
- Abramovich T. I., Vereshchaka I. V., Tal’nov A. M., Gorkovenko A. V., Dornovski M., Kostyukov A. I. (2015). Coordination of Activity of the Shoulder Belt and Shoulder Muscles in Humans During Bimanual Synchronous Two-Joint Movements. Neurophysiology 47, 310–321. 10.1007/s11062-015-9538-6 - DOI
-
- Bernstein N. A. (1967). The Coordination and Regulation of Movements. London: Pergamon Press.
LinkOut - more resources
Full Text Sources

