Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles
- PMID: 35665306
- PMCID: PMC9165723
- DOI: 10.1038/s43587-021-00143-2
Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles
Abstract
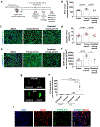
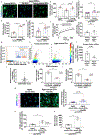
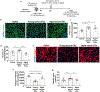
Heterochronic blood exchange (HBE) has demonstrated that circulating factors restore youthful features to aged tissues. However, the systemic mediators of those rejuvenating effects remain poorly defined. We show here that the beneficial effect of young blood on aged muscle regeneration was diminished when serum was depleted of extracellular vesicles (EVs). Whereas EVs from young animals rejuvenate aged cell bioenergetics and skeletal muscle regeneration, aging shifts EV subpopulation heterogeneity and compromises downstream benefits on recipient cells. Machine learning classifiers revealed that aging shifts the nucleic acid, but not protein, fingerprint of circulating EVs. Alterations in sub-population heterogeneity were accompanied by declines in transcript levels of the pro-longevity protein, α-Klotho, and injection of EVs improved muscle regeneration in a Klotho mRNA-dependent manner. These studies demonstrate that EVs play a key role in the rejuvenating effects of HBE and that Klotho transcripts within EVs phenocopy the effects of young serum on aged skeletal muscle.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures






Comment in
-
Young extracellular vesicles rejuvenate aged muscle.Nat Aging. 2021 Dec;1(12):1078-1080. doi: 10.1038/s43587-021-00153-0. Nat Aging. 2021. PMID: 37117521 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

