ECG Evaluation as Part of the Clinical Pharmacology Strategy in the Development of New Drugs: A Review of Current Practices and Opportunities Based on Five Case Studies
- PMID: 35665514
- PMCID: PMC9796926
- DOI: 10.1002/jcph.2095
ECG Evaluation as Part of the Clinical Pharmacology Strategy in the Development of New Drugs: A Review of Current Practices and Opportunities Based on Five Case Studies
Abstract
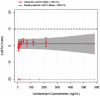
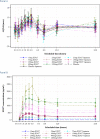
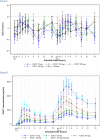
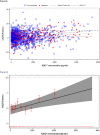
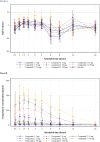
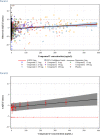
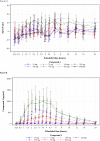
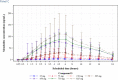
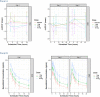
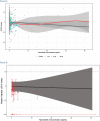
The International Conference on Harmonization (ICH) E14 document was revised in 2015 to allow concentration-corrected QT interval (C-QTc) analysis to be applied to data from early clinical pharmacology studies to exclude a small drug-induced effect on QTc. Provided sufficiently high concentrations of the drug are obtained in the first-in-human (FIH) study, this approach can be used to obviate the need for a designated thorough QT (TQT) study. The E14 revision has resulted in a steady reduction in the number of TQT studies and an increased use of FIH studies to evaluate electrocardiogram (ECG) effects of drugs in development. In this review, five examples from different sponsors are shared in which C-QTc analysis was performed on data from FIH studies. Case 1 illustrates a clearly negative C-QTc evaluation, despite observations of QTc prolongation at high concentrations in nonclinical studies. In case 2 C-QTc analysis of FIH data was performed prior to full pharmacokinetic characterization in patients, and the role of nonclinical assays in an integrated risk assessment is discussed. Case 3 illustrates a positive clinical C-QTc relationship, despite negative nonclinical assays. Case 4 demonstrates a strategy for characterizing the C-QTc relationship for a nonracemic therapy and formulation optimization, and case 5 highlights an approach to perform a preliminary C-QTc analysis early in development and postpone the definitive analysis until proof of efficacy is demonstrated. The strategy of collecting and storing ECG data from FIH studies to enable an informed decision on whether and when to apply C-QTc analysis to obviate the need for a TQT study is described.
Keywords: E14; ICH; QT; clinical pharmacology; exposure response analysis; healthy subjects.
© 2022 The Authors. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
BD is Chief Scientific Officer of Clario and owns stock and is eligible for stock options in Clario. MB is a paid consultant for Theravance Biopharma.
Figures
Similar articles
-
The New S7B/E14 Q&A Document Provides Additional Opportunities to Replace the Thorough QT Study.J Clin Pharmacol. 2023 Nov;63(11):1256-1274. doi: 10.1002/jcph.2309. Epub 2023 Aug 2. J Clin Pharmacol. 2023. PMID: 37455487
-
Time for a Fully Integrated Nonclinical-Clinical Risk Assessment to Streamline QT Prolongation Liability Determinations: A Pharma Industry Perspective.Clin Pharmacol Ther. 2021 Feb;109(2):310-318. doi: 10.1002/cpt.2029. Epub 2020 Sep 24. Clin Pharmacol Ther. 2021. PMID: 32866317 Free PMC article. Review.
-
Best practice considerations for nonclinical in vivo cardiovascular telemetry studies in non-rodent species: Delivering high quality QTc data to support ICH E14/S7B Q&As.J Pharmacol Toxicol Methods. 2023 Sep-Oct;123:107270. doi: 10.1016/j.vascn.2023.107270. Epub 2023 May 8. J Pharmacol Toxicol Methods. 2023. PMID: 37164235
-
The prospective IQ-CSRC trial: A prototype early clinical proarrhythmia assessment investigation for replacing the ICH E14 thorough QTc (TQT) study.J Pharmacol Toxicol Methods. 2016 Jul-Aug;80:1-8. doi: 10.1016/j.vascn.2016.02.181. Epub 2016 Mar 9. J Pharmacol Toxicol Methods. 2016. PMID: 26903171
-
Improving the in Vivo QTc assay: The value of implementing best practices to support an integrated nonclinical-clinical QTc risk assessment and TQT substitute.J Pharmacol Toxicol Methods. 2023 May-Jun;121:107265. doi: 10.1016/j.vascn.2023.107265. Epub 2023 Mar 28. J Pharmacol Toxicol Methods. 2023. PMID: 36997076 Review.
Cited by
-
Concentration-QTc and cardiac safety analysis of single and multiple zavegepant nasal spray doses in healthy participants to support approval.CPT Pharmacometrics Syst Pharmacol. 2024 Jun;13(6):1044-1054. doi: 10.1002/psp4.13140. Epub 2024 May 29. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 38812357 Free PMC article. Clinical Trial.
-
Pridopidine Does Not Significantly Prolong the QTc Interval at the Clinically Relevant Therapeutic Dose.Neurol Ther. 2023 Apr;12(2):597-617. doi: 10.1007/s40120-023-00449-w. Epub 2023 Feb 22. Neurol Ther. 2023. PMID: 36811812 Free PMC article.
-
FDA's insights: implementing new strategies for evaluating drug-induced QTc prolongation.J Pharmacokinet Pharmacodyn. 2025 Jun 25;52(4):37. doi: 10.1007/s10928-025-09985-4. J Pharmacokinet Pharmacodyn. 2025. PMID: 40560402 Free PMC article.
References
-
- ICH E14 . The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs. https://www.ich.org/page/efficacy-guidelines. Accessed June 27, 2022.
-
- ICH E14 . Questions & Answers (R3) December 10, 2015. https://www.ich.org/page/efficacy‐guidelines. Accessed June 27, 2022.
-
- Garnett C, Beasley N, Bhattaram VA, et al. Concentration‐QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin. Pharmacol. 2008;48:13–18. - PubMed
-
- Darpo B, Benson C, Dota C, et al. Results from the IQ‐CSRC prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin Pharmacol Ther. 2015;97:326–335. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

