Detection and Stability of SARS-CoV-2 in Three Self-Collected Specimen Types: Flocked Midturbinate Swab (MTS) in Viral Transport Media, Foam MTS, and Saliva
- PMID: 35665629
- PMCID: PMC9241800
- DOI: 10.1128/spectrum.01033-22
Detection and Stability of SARS-CoV-2 in Three Self-Collected Specimen Types: Flocked Midturbinate Swab (MTS) in Viral Transport Media, Foam MTS, and Saliva
Abstract
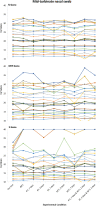
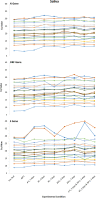
Respiratory specimen collection materials shortages hampers severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing. We compared specimen alternatives and evaluated SARS-CoV-2 RNA stability under simulated shipping conditions. We compared concordance of RT-PCR detection of SARS-CoV-2 from flocked midturbinate swabs (MTS) in viral transport media (VTM), foam MTS without VTM, and saliva. Specimens were collected between August 2020 and April 2021 from three prospective cohorts. We compared RT-PCR cycle quantification (Cq) for Spike (S), Nucleocapsid (N), and the Open Reading Frame 1ab (ORF) genes for flocked MTS and saliva specimens tested before and after exposure to a range of storage temperatures (4-30°C) and times (2, 3, and 7 days). Of 1,900 illnesses with ≥2 specimen types tested, 335 (18%) had SARS-CoV-2 detected in ≥1 specimen; 304 (91%) were concordant across specimen types. Among illnesses with SARS-CoV-2 detection, 97% (95% confidence interval [CI]: 94-98%) were positive on flocked MTS, 99% (95% CI: 97-100%) on saliva, and 89% (95% CI: 84-93%) on foam MTS. SARS-CoV-2 RNA was detected in flocked MTS and saliva stored up to 30°C for 7 days. All specimen types provided highly concordant SARS-CoV-2 results. These findings support a range of viable options for specimen types, collection, and transport methods that may facilitate SARS-CoV-2 testing during supply and personnel shortages. IMPORTANCE Findings from this analysis indicate that (1) self-collection of flocked and foam MTS and saliva samples is feasible in both adults and children, (2) foam MTS with VTM and saliva are both viable and reasonable alternatives to traditional flocked MTS in VTM for SARS-CoV-2 detection, and (3) these sample types may be stored and transported at ambient temperatures for up to 7 days without compromising sample quality. These findings support methods of sample collection for SARS-CoV-2 detection that may facilitate widespread community testing in the setting of supply and personnel shortages during the current pandemic.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; respiratory specimens; sensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Comparison of Saliva and Midturbinate Swabs for Detection of SARS-CoV-2.Microbiol Spectr. 2022 Apr 27;10(2):e0012822. doi: 10.1128/spectrum.00128-22. Epub 2022 Mar 21. Microbiol Spectr. 2022. PMID: 35311575 Free PMC article.
-
Comparative evaluation of saliva and nasopharyngeal swab for SARS-CoV-2 detection using RT-qPCR among COVID-19 suspected patients at Jigjiga, Eastern Ethiopia.PLoS One. 2023 Mar 13;18(3):e0282976. doi: 10.1371/journal.pone.0282976. eCollection 2023. PLoS One. 2023. PMID: 36913377 Free PMC article.
-
Validation of saliva sampling as an alternative to oro-nasopharyngeal swab for detection of SARS-CoV-2 using unextracted rRT-PCR with the Allplex 2019-nCoV assay.J Med Microbiol. 2021 Aug;70(8):001404. doi: 10.1099/jmm.0.001404. J Med Microbiol. 2021. PMID: 34369860 Free PMC article.
-
Dacron swab and PBS are acceptable alternatives to flocked swab and viral transport media for SARS-CoV-2.Diagn Microbiol Infect Dis. 2021 Jan;99(1):115209. doi: 10.1016/j.diagmicrobio.2020.115209. Epub 2020 Sep 16. Diagn Microbiol Infect Dis. 2021. PMID: 33080426 Free PMC article.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
Cited by
-
Performance evaluation of a new on-demand molecular test for the rapid identification of severe acute respiratory syndrome coronavirus 2 in pediatric and adult patients.Front Microbiol. 2022 Nov 3;13:999783. doi: 10.3389/fmicb.2022.999783. eCollection 2022. Front Microbiol. 2022. PMID: 36406396 Free PMC article.
References
-
- Association of State and Territorial Health Officials, AoPHL, and Council of State and Territorial Epidemiologists. 2020. COVID-19 testing needs to be limited to priority groups until sufficient testing supplies and personal protective equipment is available nationwide. Association of State and Territorial Health Officials, Arlington, VA.
-
- O’donnell C. U.S. COVID-19 tests again in short supply as infections soar, schools reopen. https://www.reuters.com/world/us/us-covid-19-tests-again-short-supply-in.... Accessed September 22, 2021.
-
- Centers for Disease Control and Prevention. Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim.... Accessed February 2, 2022.
-
- U.S. Food & Drug Administration. Emergency Use Authorization (EUA) summary color COVID-19 self-swab collection kit. https://www.fda.gov/media/141797/download. Accessed December 1, 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

