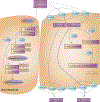
PharmGKB summary: acyclovir/ganciclovir pathway
- PMID: 35665708
- PMCID: PMC9179945
- DOI: 10.1097/FPC.0000000000000474
PharmGKB summary: acyclovir/ganciclovir pathway
Conflict of interest statement
Conflicts of interest
There are no conflicts of interest.
Figures
References
-
- Food and Drug Administration. Zovirax® (acyclovir sodium for injection, for intravenous infusion only). Product information. 1998. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/1998/18603s19lbl.pdf. Accessed 3/24/2022.
-
- Food and Drug Administration. Ganciclovir injection for intravenous use (highlights of prescribing information). 1989, revised February 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209347lbl.pdf. Accessed 3/24/2022.
-
- Crumpacker CS. Ganciclovir. N Engl J Med 1996; 335:721–729. - PubMed
-
- Zhang SM, Rehling D, Jemth AS, Throup A, Landázuri N, Almlöf I, et al. NUDT15-mediated hydrolysis limits the efficacy of anti-HCMV drug ganciclovir. Cell Chem Biol 2021; 28:1693–1702.e1696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources