Guaiac-based faecal occult blood tests versus faecal immunochemical tests for colorectal cancer screening in average-risk individuals
- PMID: 35665911
- PMCID: PMC9169237
- DOI: 10.1002/14651858.CD009276.pub2
Guaiac-based faecal occult blood tests versus faecal immunochemical tests for colorectal cancer screening in average-risk individuals
Abstract
Background: Worldwide, many countries have adopted colorectal cancer (CRC) screening programmes, often based on faecal occult blood tests (FOBTs). CRC screening aims to detect advanced neoplasia (AN), which is defined as CRC or advanced adenomas. FOBTs fall into two categories based on detection technique and the detected blood component: qualitative guaiac-based FOBTs (gFOBTs) and faecal immunochemical tests (FITs), which can be qualitative and quantitative. Screening with gFOBTs reduces CRC-related mortality.
Objectives: To compare the diagnostic test accuracy of gFOBT and FIT screening for detecting advanced colorectal neoplasia in average-risk individuals.
Search methods: We searched CENTRAL, MEDLINE, Embase, BIOSIS Citation Index, Science Citation Index Expanded, and Google Scholar. We searched the reference lists and PubMed-related articles of included studies to identify additional studies.
Selection criteria: We included prospective and retrospective studies that provided the number of true positives, false positives, false negatives, and true negatives for gFOBTs, FITs, or both, with colonoscopy as reference standard. We excluded case-control studies. We included studies in which all participants underwent both index test and reference standard ("reference standard: all"), and studies in which only participants with a positive index test underwent the reference standard while participants with a negative test were followed for at least one year for development of interval carcinomas ("reference standard: positive"). The target population consisted of asymptomatic, average-risk individuals undergoing CRC screening. The target conditions were CRC and advanced neoplasia (advanced adenomas and CRC combined).
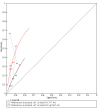
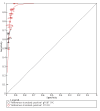
Data collection and analysis: Two review authors independently screened and selected studies for inclusion. In case of disagreement, a third review author made the final decision. We used the Rutter and Gatsonis hierarchical summary receiver operating characteristic model to explore differences between tests and identify potential sources of heterogeneity, and the bivariate hierarchical model to estimate sensitivity and specificity at common thresholds: 10 µg haemoglobin (Hb)/g faeces and 20 µg Hb/g faeces. We performed indirect comparisons of the accuracy of the two tests and direct comparisons when both index tests were evaluated in the same population.
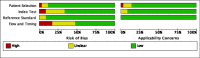
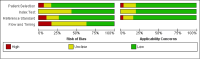
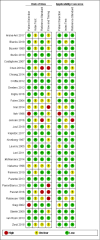
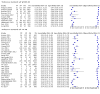
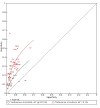
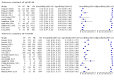
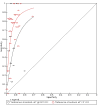
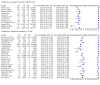
Main results: We ran the initial search on 25 June 2019, which yielded 63 studies for inclusion. We ran a top-up search on 14 September 2021, which yielded one potentially eligible study, currently awaiting classification. We included a total of 33 "reference standard: all" published articles involving 104,640 participants. Six studies evaluated only gFOBTs, 23 studies evaluated only FITs, and four studies included both gFOBTs and FITs. The cut-off for positivity of FITs varied between 2.4 μg and 50 µg Hb/g faeces. For each Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 domain, we assessed risk of bias as high in less than 20% of studies. The summary curve showed that FITs had a higher discriminative ability than gFOBTs for AN (P < 0.001) and CRC (P = 0.004). For the detection of AN, the summary sensitivity of gFOBTs was 15% (95% confidence interval (CI) 12% to 20%), which was significantly lower than FITs at both 10 μg and 20 μg Hb/g cut-offs with summary sensitivities of 33% (95% CI 27% to 40%; P < 0.001) and 26% (95% CI 21% to 31%, P = 0.002), respectively. Results were simulated in a hypothetical cohort of 10,000 screening participants with 1% CRC prevalence and 10% AN prevalence. Out of 1000 participants with AN, gFOBTs missed 850, while FITs missed 670 (10 μg Hb/g cut-off) and 740 (20 μg Hb/g cut-off). No significant differences in summary specificity for AN detection were found between gFOBTs (94%; 95% CI 92% to 96%), and FITs at 10 μg Hb/g cut-off (93%; 95% CI 90% to 95%) and at 20 μg Hb/g cut-off (97%; 95% CI 95% to 98%). So, among 9000 participants without AN, 540 were offered (unnecessary) colonoscopy with gFOBTs compared to 630 (10 μg Hb/g) and 270 (20 μg Hb/g) with FITs. Similarly, for the detection of CRC, the summary sensitivity of gFOBTs, 39% (95% CI 25% to 55%), was significantly lower than FITs at 10 μg and 20 μg Hb/g cut-offs: 76% (95% CI 57% to 88%: P = 0.001) and 65% (95% CI 46% to 80%; P = 0.035), respectively. So, out of 100 participants with CRC, gFOBTs missed 61, and FITs missed 24 (10 μg Hb/g) and 35 (20 μg Hb/g). No significant differences in summary specificity for CRC were found between gFOBTs (94%; 95% CI 91% to 96%), and FITs at the 10 μg Hb/g cut-off (94%; 95% CI 87% to 97%) and 20 μg Hb/g cut-off (96%; 95% CI 91% to 98%). So, out of 9900 participants without CRC, 594 were offered (unnecessary) colonoscopy with gFOBTs versus 594 (10 μg Hb/g) and 396 (20 μg Hb/g) with FITs. In five studies that compared FITs and gFOBTs in the same population, FITs showed a higher discriminative ability for AN than gFOBTs (P = 0.003). We included a total of 30 "reference standard: positive" studies involving 3,664,934 participants. Of these, eight were gFOBT-only studies, 18 were FIT-only studies, and four studies combined both gFOBTs and FITs. The cut-off for positivity of FITs varied between 5 µg to 250 µg Hb/g faeces. For each QUADAS-2 domain, we assessed risk of bias as high in less than 20% of studies. The summary curve showed that FITs had a higher discriminative ability for detecting CRC than gFOBTs (P < 0.001). The summary sensitivity for CRC of gFOBTs, 59% (95% CI 55% to 64%), was significantly lower than FITs at the 10 μg Hb/g cut-off, 89% (95% CI 80% to 95%; P < 0.001) and the 20 μg Hb/g cut-off, 89% (95% CI 85% to 92%; P < 0.001). So, in the hypothetical cohort with 100 participants with CRC, gFOBTs missed 41, while FITs missed 11 (10 μg Hb/g) and 11 (20 μg Hb/g). The summary specificity of gFOBTs was 98% (95% CI 98% to 99%), which was higher than FITs at both 10 μg and 20 μg Hb/g cut-offs: 94% (95% CI 92% to 95%; P < 0.001) and 95% (95% CI 94% to 96%; P < 0.001), respectively. So, out of 9900 participants without CRC, 198 were offered (unnecessary) colonoscopy with gFOBTs compared to 594 (10 μg Hb/g) and 495 (20 μg Hb/g) with FITs. At a specificity of 90% and 95%, FITs had a higher sensitivity than gFOBTs.
Authors' conclusions: FITs are superior to gFOBTs in detecting AN and CRC in average-risk individuals. Specificity of both tests was similar in "reference standard: all" studies, whereas specificity was significantly higher for gFOBTs than FITs in "reference standard: positive" studies. However, at pre-specified specificities, the sensitivity of FITs was significantly higher than gFOBTs.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
EG has no interests to disclose.
PW has no interests to disclose.
ES has no interests to disclose.
AR has no interests to disclose.
LD has no interests to disclose.
AZ has no interests to disclose.
IL was involved in a published overview paper on risk‐based CRC screening in
WB has no interests to disclose.
SB has no interests to disclose.
JD has a Cochrane Diagnostic Test Accuracy (DTA) Editorial role but was not involved in the editorial process. JD was involved in published opinions in medical journals, the public press, broadcast, and social media relevant to the interventions in the work: eight podcasts, including Talk Evidence (BMJ), More or Less (Radio 4), Inside Science (Radio 4), and The Newscast (Radio 4); five opinion pieces in
ES received payment for a book:
ML has no interests to disclose.
MS has no interests to disclose.
EK has no interests to disclose.
Figures














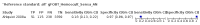

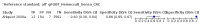
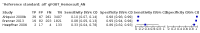
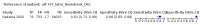





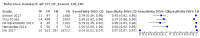
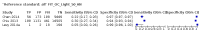

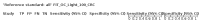
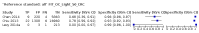
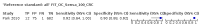
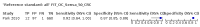


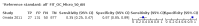
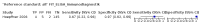

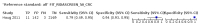


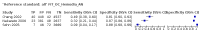



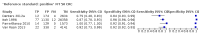


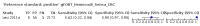




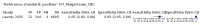
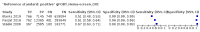

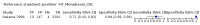
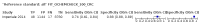
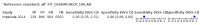
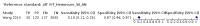
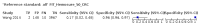
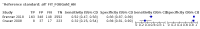
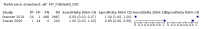
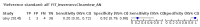
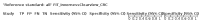

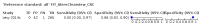
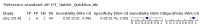
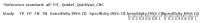
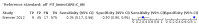
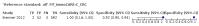
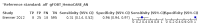
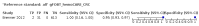
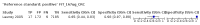
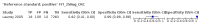


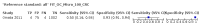
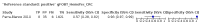
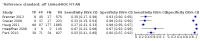
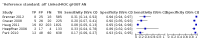
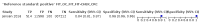


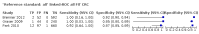

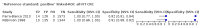
Comment in
-
Guaiac-Based FOBTs vs. FITs for Colorectal Cancer Screening in Average-Risk Adults.Am Fam Physician. 2023 Feb;107(2):134-135. Am Fam Physician. 2023. PMID: 36791449 No abstract available.
References
References to studies included in this review
Ahlquist 2008a {published data only}
Ahlquist 2008b {published data only}
Aniwan 2015 {published data only}
-
- Aniwan S, Rerknimitr R, Kongkam P, Wisedopas N, Ponuthai Y, Chaithongrat S, et al.A combination of clinical risk stratification and fecal immunochemical test results to prioritize colonoscopy screening in asymptomatic participants. Gastrointestinal Endoscopy 2015;81(3):719-27. - PubMed
Aniwan 2017 {published data only}
-
- Aniwan S, Ratanachu Ek T, Pongprasobchai S, Limsrivilai J, Praisontarangkul OA, Pisespongsa P, et al.The optimal cut-off level of the fecal immunochemical test for colorectal cancer screening in a country with limited colonoscopy resources: a multi-center study from Thailand. Asian Pacific Journal of Cancer Prevention 2017;18(2):405-12. - PMC - PubMed
Arana‐Arri 2017a {published and unpublished data}
Arana‐Arri 2017b {published data only}
Blanks 2019 {published and unpublished data}
-
- Blanks R, Burón Pust A, Alison R, He E, Barnes I, Patnick J, et al.Screen-detected and interval colorectal cancers in England: associations with lifestyle and other factors in women in a large UK prospective cohort. International Journal of Cancer 2019;145(3):728-34. [DOI: 10.1002/ijc.32168] - DOI - PMC - PubMed
Bouvier 1999 {published data only}
Brenner 2012 {published data only}
-
- Brenner G, Faure H, Heuer S, Reinholz J.Detection of colorectal findings for cancer prevention by immunochemical stool test with different sensitivity levels [Detektion von kolorektalen Befunden zur Darmkrebsprävention mit immunologischem Stuhltest in verschiedenen Empfindlichkeitsstufen]. Zeitschrift fur Gastroenterologie 2012;50(10):1083-8. - PubMed
Brenner 2013 {published data only (unpublished sought but not used)}
-
- Brenner H, Tao S.Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. European Journal of Cancer 2013;49(14):3049-54. - PubMed
Brenner 2018 {published data only}
Burón 2019 {published and unpublished data}
-
- Burón A, Macià F, Andreu M, Pellisé M, Castells X, Grau J.Population-based colorectal cancer screening: interval cancers and relationship with the quantitative faecal immunological for hemoglobin. Medica Clinica 2019;152(8):303-6. - PubMed
Castiglione 2007 {published data only}
Chang 2017 {published data only}
-
- Chang LC, Shun CT, Hsu WF, Tu CH, Tsai PY, Lin BR, et al.Fecal immunochemical test detects sessile serrated adenomas and polyps with a low level of sensitivity. Clinical Gastroenteroly and Hepatology 2017;15(6):872-9. - PubMed
Chen 2014 {published data only}
-
- Chen YY, Chen TH, Su MY, Ning HC, Kuo CJ, Lin WP, et al.Accuracy of immunochemical fecal occult blood test for detecting colorectal neoplasms in individuals undergoing health check-ups. Advances in Digestive Medicine 2014;1:74-9.
Chen 2016a {published data only}
Cheng 2002 {published and unpublished data}
-
- Cheng TI, Wong JM, Hong CF, Cheng SH, Cheng TJ, Shieh MJ, et al.Colorectal cancer screening in asymptomatic adults: comparison of colonoscopy, sigmoidoscopy and fecal occult blood tests. Journal of the Formosan Medical Association 2002;101(10):685-90. - PubMed
Chiang 2014a {published data only}
-
- Chiang TH, Chuangs SL, Chen SL, Chiu HM, Yen AM, Chiu SY, et al.Difference in performance of fecal immunochemical tests with the same hemoglobin cutoff concentration in a nationwide colorectal cancer screening program. Gastroenterology 2014;147(6):1317-26. - PubMed
Chiang 2014b {published data only}
-
- Chiang TH, Chuangs SL, Chen SL, Chiu HM, Yen AM, Chiu SY, et al.Difference in performance of fecal immunochemical tests with the same hemoglobin cutoff concentration in a nationwide colorectal cancer screening program. Gastroenterology 2014;147(6):1317-26. - PubMed
Chiu 2013 {published data only}
-
- Chiu HM, Lee YC, Tu CH, Chen CC, Tseng PH, Liang JT, et al.Association between early stage colon neoplasms and false-negative results from the fecal immunochemical test. Clinical Gastroenterology and Hepatology 2013;11(7):832-8. - PubMed
Chiu 2016a {published data only}
-
- Chiu HM, Ching JY, Wu KC, Rerknimitr R, Li J, Wu DC, et al.A risk-scoring system combined with a fecal immunochemical test is effective in screening high-risk subjects for early colonoscopy to detect advanced colorectal neoplasms. Gastroenterology 2016;150(3):617-25. - PubMed
Crotta 2012 {published data only}
-
- Crotta S, Segnan N, Paganin S, Dagnes B, Rosset R, Senore C.High rate of advanced adenoma detection in 4 rounds of colorectal cancer screening with the fecal immunochemical test. Clinical Gastroenterology and Hepatology 2012;10(6):633-8. - PubMed
Cruz‐Correa 2007 {published data only}
-
- Cruz-Correa M, Schultz K, Jagannath S, Harris M, Kantsevoy S, Bedine M, et al.Performance characteristics and comparison of two fecal occult blood tests in patients undergoing colonoscopy. Digestive Diseases and Sciences 2007;52(4):1009-13. - PubMed
Denters 2012a {published data only}
-
- Denters MJ, Deutekom M, Bossuyt PM, Stroobants AK, Fockens P, Dekker E.Lower risk of advanced neoplasia among patients with a previous negative result from a fecal test for colorectal cancer. Gastroenterology 2012;142(3):497-504. - PubMed
Denters 2012b {published data only}
-
- Denters MJ, Deutekom M, Bossuyt PM, Stroobants AK, Fockens P, Dekker E.Lower risk of advanced neoplasia among patients with a previous negative result from a fecal test for colorectal cancer. Gastroenterology 2012;142(3):497-504. - PubMed
De Wijkerslooth 2012 {published data only}
-
- De Wijkerslooth TR, Stoop EM, Bossuyt PM, Meijer GA, Van Ballegooijen M, Van Roon AH, et al.Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. American Journal of Gastroenterology 2012;107(10):1570-8. - PubMed
Digby 2016 {published data only}
-
- Digby J, Fraser CG, Carey FA, Lang J, Stanners G, Steele RJ.Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited. Journal of Medical Screening 2016;23(3):130-4. - PubMed
Faivre 2004 {published data only}
-
- Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, et al.Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology 2004;126(7):1674-80. - PubMed
Giai 2014 {published and unpublished data}
-
- Giai J, Exbrayat C, Boussat B, Poncet F, Bureau du ColombierP, Colonna M, et al.Sensitivity of a colorectal cancer screening program based on a guaiac test: a population-based study. Clinics and Research in Hepatology and Gastroenterology 2014;38(1):106-11. [PMID: ] - PubMed
Graser 2009 {published data only}
-
- Graser A, Stieber P, Nagel D, Schäfer C, Horst D, Becker CR, et al.Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut 2009;58(2):241-8. - PubMed
Haug 2011 {published data only}
Hernandez 2014 {published data only}
Hoepffner 2006 {published data only}
-
- Hoepffner N, Shastri YM, Hanisch E, Rösch W, Mössners J, Caspary WF, et al.Comparative evaluation of a new bedside faecal occult blood test in a prospective multicentre study. Alimentary Pharmacology and Therapeutics 2006;23(1):145-54. - PubMed
Imperiale 2004 {published data only}
-
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME.Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. New England Journal of Medicine 2004;351(26):2704-14. - PubMed
Imperiale 2014 {published data only}
-
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al.Multitarget stool DNA testing for colorectal-cancer screening. New England Journal of Medicine 2014;370(14):1287-97. - PubMed
Itoh 1996 {published data only}
-
- Itoh M, Takahashi K, Nishida H, Sakagami K, Okubo T.Estimation of the optimal cut off point in a new immunological faecal occult blood test in a corporate colorectal cancer screening programme. Journal of Medical Screening 1996;3(2):66-71. - PubMed
Jensen 2016 {published and unpublished data}
Juul 2018 {published data only}
-
- Juul JS, Andersen B, Laurberg S, Carlsen AH, Olesen F, Vedsted P.Differences in diagnostic activity in general practice and findings for individuals invited to the Danish screening programme for colorectal cancer: a population-based cohort study. Scandinvian Journal of Primary Health Care 2018;36(3):281-90. - PMC - PubMed
Kapidzic 2017 {published data only}
-
- Kapidzic A, Van Roon AH, Van Leerdam ME, Van Vuuren AJ, Van Ballegooijen M, Lansdorp-Vogelaar I, et al.Attendance and diagnostic yield of repeated two-sample faecal immunochemical test screening for colorectal cancer. Gut 2017;66(1):118-23. - PubMed
Khalid‐de Bakker 2011 {published data only}
-
- Khalid-de Bakker CA, Jonkers DM, Sanduleanu S, Bruïne AP, Meijer GA, Janssen JB, et al.Test performance of immunologic fecal occult blood testing and sigmoidoscopy compared with primary colonoscopy screening for colorectal advanced adenomas. Cancer Prevention Research 2011;4(10):1563-71. - PubMed
Kim 2017 {published data only}
Kronborg 1987 {published data only}
-
- Kronborg O, Fenger C, Søndergaard O, Pedersen KM, Olsen J.Initial mass screening for colorectal cancer with fecal occult blood test. A prospective randomized study at Funen in Denmark. Scandinavion Journal of Gastroenterology 1987;2(6):677-86. - PubMed
Launoy 2005 {published data only}
-
- Launoy GD, Bertrand HJ, Berchi C.Evaluation of an immunochemical fecal occult blood test with automated reading in screening for colorectal cancer in a general average‐risk population. International Journal of Cancer 2005;115(3):493-96. - PubMed
Levi 2011a {published data only}
-
- Levi Z, Birkenfeld S, Vilkin A, Bar-Chana M, Lifshitz I, Chared M, et al.A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. International Journal of Cancer 2011;128(10):2415-24. - PubMed
Levi 2011b {published data only}
-
- Levi Z, Birkenfeld S, Vilkin A, Bar-Chana M, Lifshitz I, Chared M, et al.A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. International Journal of Cancer 2011;128(10):2415-24. - PubMed
Levy 2014a {published and unpublished data}
Levy 2014b {published data only}
Levy 2014c {published data only}
Levy 2014d {published data only}
Liebermann 2001 {published data only}
-
- Lieberman DA, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, et al.One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. New England Journal of Medicine 2001;345(8):555-60. - PubMed
McNamara 2014 {published data only}
-
- McNamara D, Leen R, Seng-Lee C, Shearer N, Crotty P, Neary P, et al.Sustained participation, colonoscopy uptake and adenoma detection rates over two rounds of the Tallaght-Trinity College colorectal cancer screening programme with the faecal immunological test. European Journal of Gastroenterology and Hepatology 2014;26(12):1415-21. [PMID: ] - PubMed
Nakama 1996 {published data only}
-
- Nakama H, Kamijo N, Abdul Fattah AS, Zhang B.Validity of immunological faecal occult blood screening for colorectal cancer: a follow up study. Journal of Medical Screening 1996;3(2):63-5. - PubMed
Nakama 2000 {published data only}
-
- Nakama H, Zhang B, Fattah AS, Zhang X.Colorectal cancer in iron deficiency anemia with a positive result on immunochemical fecal occult blood. International Journal of Colorectal Disease 2000;15(5-6):271-4. - PubMed
Nakazato 2006 {published and unpublished data}
-
- Nakazato M, Yamano H, Matsushita H, Sato K, Fujita K, Yamanaka Y, et al.Immunologic fecal occult blood test for colorectal cancer screening. Japan Medical Association Journal 2006;49:203-7.
Omata 2011 {published data only}
-
- Omata F, Shintani A, Isozaki M, Masuda K, Fujita Y, Fukui T.Diagnostic performance of quantitative fecal immunochemical test and multivariate prediction model for colorectal neoplasms in asymptomatic individuals. European Journal of Gastroenterology and Hepatology 2011;23(11):1036-41. - PubMed
Paimela 2010 {published data only}
-
- Paimela H, Malila N, Palva T, Hakulinen T, Vertio H, Jarvinen H.Early detection of colorectal cancer with faecal occult blood test screening. British Journal of Surgery 2010;97(10):1567-71. - PubMed
Parente 2013 {published data only}
-
- Parente F, Boemo C, Ardizzoia A, Costa M, Carzaniga P, Ilardo A, et al.Outcomes and cost evaluation of the first two rounds of a colorectal cancer screening program based on immunochemical fecal occult blood test in northern Italy. Endoscopy 2013;45(1):27-34. - PubMed
Park 2010 {published data only}
-
- Park DI, Ryu S, Kim YH, Lee SH, Lee CK, Eun CS, et al.Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. American Journal of Gastroenterology 2010;105(9):2017-25. - PubMed
Parra‐Blanco 2010 {published data only}
-
- Parra-Blanco A, Gimeno-Garcia AZ, Quintero E, Nicolas D, Moreno SG, Jimenez A, et al.Diagnostic accuracy of immunochemical versus guaiac faecal occult blood tests for colorectal cancer screening. Journal of Gastroenterology 2010;45(7):703-12. - PubMed
Paszat 2016 {published data only}
Ribbing Wilen 2019 {published data only}
-
- Ribbing Wilen H, Blom J, Hoijer J, Andersson G, Lowbeer C, Hultcrantz R.Fecal immunochemical test in cancer screening - colonoscopy outcome in FIT positives and negatives. Scandinavian Journal of Gastroenterology 2019;54(3):303-10. - PubMed
Robinson 1996 {published data only}
-
- Robinson MH, Marks CG, Farrands PA, Bostock K, Hardcastle JD.Screening for colorectal cancer with an immunological faecal occult blood test: 2-year follow-up. British Journal of Surgery 1996;83(4):500-1. - PubMed
Sieg 2002 {published data only}
-
- Sieg A, Wirth A, Luthgens K, Schmidt-Gayk H.Six years of screening for colorectal neoplasms with an immunological fecal occult hemoglobin test. Verdauungskrankheiten 2002;20:114-7.
Siripongpreeda 2016 {published data only}
Sohn 2005 {published data only}
Steele 2009 {published data only}
-
- Steele RJ, McClements PL, Libby G, Black R, Morton C, Birrell J, et al.Results from the first three rounds of the Scottish demonstration pilot of FOBT screening for colorectal cancer. Gut 2009;58(4):530-5. - PubMed
Sung 2003 {published data only}
-
- Sung JJ, Chan FK, Leung WK, Wu JC, Lau JY, Ching J, et al.Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology 2003;124(3):608-14. - PubMed
Van Roon 2013 {published data only}
-
- Van Roon AH, Goede SL, Van Ballegooijen M, Van Vuuren AJ, Looman CW, Biermann K, et al.Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut 2013;62(3):409-15. [PMID: ] - PubMed
Wong 2014 {published data only}
Wu 2014 {published data only}
-
- Wu T, Kuo K, Wu Y, Lin K.Diagnostic accuracy of a single qualitative immunochemical fecal occult blood test coupled with physical measurements. Chinese Medical Journal 2014;127(24):4164-70. - PubMed
Zorzi 2018 {published and unpublished data}
-
- Zorzi M, Hassan C, Capodaglio G, Narne E, Turrin A, Baracco M, et al.Divergent long-term detection rates of proximal and distal advanced neoplasia in fecal immunochemical test screening programs: a retrospective cohort study. Annals of Internal Medicine 2018;169(9):602-9. - PubMed
References to studies excluded from this review
Allison 1996 {published data only}
-
- Allison JE, Tekawa IS, Ransom LJ, Adrain AL.A comparison of fecal occult-blood tests for colorectal-cancer screening. New England Journal of Medicine 1996;334(3):155-9. - PubMed
Brenner 2010a {published data only}
-
- Brenner G, Faure H, Reinholz J.Comparison of the guaiac-based test (g-FOBT) and the immunochemical test (i-FOBT) with the results of the screening colonoscopy in an asymptomatic population. Verdauungskrankheiten 2010;28.
Brenner 2010b {published data only}
-
- Brenner H, Haug U, Hundt S.Sex differences in performance of fecal occult blood testing. American Journal of Gastroenterology 2010;105(11):2457-64. - PubMed
Brenner 2014 {published data only}
-
- Brenner H, Hoffmeister M, Birkner B, Stock C.Diagnostic performance of guaiac-based fecal occult blood test in routine screening: state-wide analysis from Bavaria, Germany. American Journal of Gastroenterology 2014;109(3):427-35. - PubMed
Castiglione 1996 {published data only}
Castiglione 2000 {published data only}
-
- Castiglione G, Zappa M, Grazzini G, Rubeca T, Turco P, Sani C, et al.Screening for colorectal cancer by faecal occult blood test: comparison of immunochemical tests. Journal of Medical Screening 2000;7(1):35-7. - PubMed
Chiu 2015 {published data only}
Crotta 2004 {published data only}
-
- Crotta S, Castiglione G, Grazzini G, Valle F, Mosconi S, Rosset R.Feasibility study of colorectal cancer screening by immunochemical faecal occult blood testing: results in a northern Italian community. European Journal of Gastroenterology and Hepatology 2004;16(1):33-7. - PubMed
Cubiella 2014 {published data only}
Cummings 1986 {published data only}
-
- Cummings KM, Michalek A, Tidings J, Herrera L, Mettlin C.Results of a public screening program for colorectal cancer. New York State Journal of Medicine 1986;86(2):68-72. - PubMed
Dancourt 2008 {published data only}
-
- Dancourt V, Lejeune C, Lepage C, Gaillaird MC, Meny B, Faivre J.Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. European Journal of Cancer 2008;44(15):2254-8. - PubMed
Elferink 2018 {published data only}
-
- Elferink MA, Toes-Zoutendijk E, Vink GR, Lansdorp-Vogelaar I, Meijer GA, Dekker E, et al.National population screening for colorectal carcinoma in the Netherlands: results of the first years since the implementation in 2014. Nederlands Tijdschrift voor Geneeskunde 2018;162:D2283. - PubMed
Faivre 2012 {published data only}
-
- Faivre J, Dancourt V, Denis B, Dorval E, Piette C, Perrin P, et al.Comparison between a guaiac and three immunochemical faecal occult blood tests in screening for colorectal cancer. European Journal of Cancer 2012;48(16):2969-76. - PubMed
Fraser 2006 {published data only}
-
- Fraser CG, Matthew CM, Mowat NA, Wilson JA, Carey FA, Steele RJ.Immunochemical testing of individuals positive for guaiac faecal occult blood test in a screening programme for colorectal cancer: an observational study. Lancet Oncology 2006;7(2):127-31. - PubMed
Gies 2018 {published data only}
-
- Gies A, Cuk K, Schrotz-King, Brenner H.Direct comparison of diagnostic performance of 9 quantitative fecal immunochemical tests for colorectal cancer screening. Gastroenterology 2018;154(1):93-104. - PubMed
Hardcastle 1996 {published data only}
-
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al.Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348(9040):1472-7. - PubMed
Hoff 2004 {published data only}
-
- Hoff G, Grotmol T, Thiis-Evensen E, Bretthauer M, Gondal G, Vatn MH.Testing for faecal calprotectin (PhiCal) in the Norwegian Colorectal Cancer Prevention trial on flexible sigmoidoscopy screening: comparison with an immunochemical test for occult blood (FlexSure OBT). Gut 2004;53(9):1329-33. - PMC - PubMed
Hol 2010 {published data only}
-
- Hol L, Van Leerdam ME, Van Ballegooijen M, Van Vuuren AJ, Van Dekken H, Reijerink JC, et al.Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010;59(1):62-8. - PubMed
Hundt 2009 {published data only}
-
- Hundt S, Haug U, Brenner H.Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Annals of Internal Medicine 2009;150(3):162-9. - PubMed
Jørgensen 2002 {published data only}
Ko 2003 {published data only}
-
- Ko CW, Dominitz JA, Nguyen TD.Fecal occult blood testing in a general medical clinic: comparison between guaiac-based and immunochemical-based tests. American Journal of Medicine 2003;115(2):111-4. - PubMed
Libby 2018 {published data only}
Mandel 1993 {published data only}
-
- Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al.Reducing mortality from colorectal cancer by screening for fecal occult blood. New England Journal of Medicine 1993;328(19):1365-71. - PubMed
Mandel 2000 {published data only}
-
- Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al.The effect of fecal occult-blood screening on the incidence of colorectal cancer. New England Journal of Medicine 2000;343(22):1603-7. - PubMed
Oort 2010 {published data only}
-
- Oort FA, Terhaar Sive Droste JS, Van der Hulst RW, Van Heukelem HA, Loffeld RJ, Wesdorp IC, et al.Colonoscopy-controlled intra-individual comparisons to screen relevant neoplasia: faecal immunochemical test vs. guaiac-based faecal occult blood test. Alimentary Pharmacology and Therapeutics 2010;31(3):432-9. - PubMed
Quintero 2012 {published data only}
-
- Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, et al.Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. New England Journal of Medicine 2012;366(8):697-706. - PubMed
Scholefield 2002 {published data only}
Steele 2013 {published data only}
Van Rossum 2008 {published data only}
-
- Van Rossum LG, Van Rijn AF, Laheij RJ, Van Oijen MG, Fockens P, Van Krieken HH, et al.Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008;135(1):82-90. - PubMed
Winawer 1980 {published data only}
-
- Winawer SJ, Andrews M, Flehinger B, Sherlock P, Schottenfeld D, Miller DG.Progress report on controlled trial of fecal occult blood testing for the detection of colorectal neoplasia. Cancer 1980;45(12):2959-64. - PubMed
Zorzi 2011 {published data only}
-
- Zorzi M, Fedato C, Grazzini G, Stocco FC, Banovich F, Bortoli A, et al.High sensitivity of five colorectal screening programmes with faecal immunochemical test in the Veneto Region, Italy. Gut 2011;60(7):944-9. - PubMed
References to studies awaiting assessment
Cheng 2021 {published data only}
-
- Cheng WC, Chen PJ, Kang JW, Chen WY, Sheu BS.Age, male sex, smoking and metabolic syndrome as risk factors of advanced colorectal neoplasia for fecal immunochemical test negative patients. Journal of the Formosan Medical Association 2021;S0929-6646(21)00240-0.:Online ahead of print.. - PubMed
Additional references
Andermann 2008
Bramer 2016
Buscemi 2006
-
- Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP.Single data extraction generated more errors than double data extraction in systematic reviews. Journal of Clinical Epidemiology 59;7:697-703. - PubMed
Carethers 2015
Ciatto 2007
Deeks 2005
-
- Deeks JJ, Macaskill P, Irwig L.The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58:882-93. - PubMed
Deeks 2013
-
- Deeks JJ, Bossuyt PM, Gatsonis C (editors).Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
Doust 2005
-
- Doust JA, Pietrzak E, Sanders S, Glasziou PP.Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. Journal of Clinical Epidemiology 2005;58(5):444-9. - PubMed
Edwards 2005
-
- Edwards JB.Screening for colorectal cancer using faecal blood testing: varying the positive cut-off value. Pathology 2005;37:565-8. - PubMed
EndNote 2015 [Computer program]
-
- EndNote.Version X7. Thomson Reuters, 2015.
Fairfield 2017
Fraser 2012
-
- Fraser CG, Allison JE, Halloran SP, Young GP, Expert Working Group on Fecal Immunochemical Tests for Hemoglobin, Colorectal Cancer Screening Committee, World Endoscopy Organization.A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. Journal of the National Cancer Institute 2012;104(11):810-4. - PubMed
Freitas 2013
Hewitson 2008
-
- Hewitson P, Glasziou P, Watson E, Towler B, Irwig L.Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (Hemoccult): an update. Americal Journal of Gastroenterology 2008;103(6):1541-9. - PubMed
Hol 2009
Imperiale 2019
-
- Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PO.Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Annals of Internal Medicine 2019;170(5):319-29. - PubMed
Johnson 2013
Konrad 2010
Kronborg 1996
-
- Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O.Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467-71. - PubMed
Kuipers 2013
-
- Kuipers EJ, Rösch T, Bretthauer M.Colorectal cancer screening--optimizing current strategies and new directions. Nature Reviews Clinical Oncology 2013;10(3):130-42. - PubMed
Lansdorp‐Vogelaar 2012
-
- Lansdorp-Vogelaar I, Karsa L, International Agency for Research on Cancer.European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Introduction. Endoscopy 2012;44(Suppl 3):SE15-30. [PMID: ] - PubMed
Launois 2014
-
- Launois R, Le Moine JG, Uzzan B, Fiestas Navarrete LI, Benamouzig R.Systematic review and bivariate/HSROC random-effect meta-analysis of immunochemical and guaiac-based fecal occult blood tests for colorectal cancer screening. European Journal of Gastroenterology and Hepatology 2014;9:978-89. - PubMed
Lee 2014
Leeflang 2008
Leeflang 2009
-
- Leeflang MM, Bossuyt PM, Irwig L.Diagnostic test accuracy may vary with prevalence: implications for evidence-based diagnosis. Journal of Clinical Epidemiology 2009;62(1):5-12. - PubMed
Levi 2007
-
- Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, et al.A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Annals of Internal Medicine 2007;146:244-55. - PubMed
Lindholm 2008
-
- Lindholm E, Brevinge H, Haglind E.Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. British Journal of Surgery 2008;95(8):1029-36. - PubMed
Macaskill 2013
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y.Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
Naaktgeboren 2016
Nieuwenburg 2019
-
- Nieuwenburg SA, Vuik FE, Kruip MJ, Kuipers EJ, Spaander MC.Effect of anticoagulants and NSAIDs on accuracy of faecal immunochemical tests (FITs) in colorectal cancer screening: a systematic review and meta-analysis. Gut 2019;68(5):866-72. - PubMed
Reitsma 2005
-
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH.Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58:982‐90. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rockey 1999
-
- Rockey D.Occult gastrointestinal bleeding. New England Journal of Medicine 1999;341:38-46. - PubMed
Rozen 2006
-
- Rozen P, Waked A, Vilkin A, Levi Z, Niv Y.Evaluation of a desk top instrument for the automated development and immunochemical quantification of fecal occult blood. Medical Science Monitor 2006;12:MT27-32. - PubMed
Rutter 2001
-
- Rutter CM, Gatsonis CA.A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20:2865‐2884. - PubMed
Sanduleanu 2014
-
- Sanduleanu S, le Clercq CM, Dekker E, Meijer GA, Rabeneck L, Rutter MD, et al.Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut 2015;64(8):1257-67. - PubMed
Schreuders 2015
-
- Schreuders EH, Ruco A, Rabeneck L, Schoen SE, Sung JJ, Young GP, et al.Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64(10):1637-49. - PubMed
Shaukat 2013
-
- Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al.Long-term mortality after screening for colorectal cancer. New England Journal of Medicine 2013;369(13):1106-14. - PubMed
Sinatra 1999
-
- Sinatra MA, St John DJ, Young GP.Interference of plant peroxidases with guaiac-based fecal occult blood tests is avoidable. Clinical Chemistry 1999;45(1):123-6. - PubMed
Song 2002
-
- Song F, Khan KS, Dinnes J, Sutton AJ.Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. International Journal of Epidemiology 2002;31:88-95. - PubMed
Spada 2014
Stata [Computer program]
-
- Stata.Version 16. College Station, TX, USA: StataCorp, 2019. Available at www.stata.com.
Takwoingi 2013
-
- Takwoingi Y, Leeflang MM, Deeks JJ.Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Annals of Internal Medicine 2013;158(7):544-54. - PubMed
Tinmouth 2015
-
- Tinmouth J, Lansdorp-Vogelaar I, Allison JE.Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know. Gut 2015;64(8):1327-37. - PubMed
Van Doorn 2015
-
- Van Doorn SC, Stegeman I, Stroobants AK, Mundt MW, De Wijkersloothet TR, Fockens P, et al.Fecal immunochemical testing results and characteristics of colonic lesions. Endoscopy 2015;47(11):1011-7. - PubMed
Van Houwelingen 2002
-
- Van Houwelingen HC, Arends LR, Stijnen T.Advanced methods in meta-analysis: multivariate approach and meta-regression. Statistics in Medicine 2002;21(4):589-624. - PubMed
Van Rijn 2006
-
- Van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, Van Deventer SJ, Dekker E.Polyp miss rate determined by tandem colonoscopy: a systematic review. American Journal of Gastroenterology 2006;101:343-50. - PubMed
Whiting 2003
Whiting 2011
Wieten 2019
-
- Wieten E, Schreuders EH, Grobbee EJ, Nieboer D, Bramer WM, Lansdorp-Vogelaar I, et al.Incidence of faecal occult blood test interval cancers in population-based colorectal cancer screening: a systematic review and meta-analysis. Gut 2019;68(5):873-81. - PubMed
Wieten 2020
-
- Wieten E, Klerk CM, Lansdorp-Vogelaar I, Bossuyt PM, Dekker E, Spaander MC.A quarter of participants with advanced neoplasia have discordant results from 2-sample fecal immunochemical tests for colorectal cancer screening. Clinical Gastroenterology and Hepatology 2020;18(8):1805-11. - PubMed
Wilschut 2011
-
- Wilschut JA, Habbema JD, Van Leerdam ME, Hol L, Lansdorp-Vogelaar I, Kuipers EJ, et al.Fecal occult blood testing when colonoscopy capacity is limited. Journal of the National Cancer Institute 2011;103(23):1741-51. - PubMed
Wilson 1968
-
- Wilson JM, Jungner YG.Principles and practice of mass screening for disease. Public Health Papers 34 1968;65:281-393. - PubMed
Winawer 1993
-
- Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al.Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. New England Journal of Medicine 1993;329:1977-81. - PubMed
Winawer 1997
-
- Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, et al.Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997;112:594-642. - PubMed
Young 2002
-
- Young GP, St John DJ, Winawer SJ, Rozen P.Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. American Journal of Gastroenterology 2002;97:2499-507. - PubMed
Young 2009
-
- Young GP.Population-based screening for colorectal cancer: Australian research and implementation. Journal of Gastroenterology and Hepatology 2009;24 Suppl 3:S33-42. - PubMed
Young 2015
Zauber 2012
Zorzi 2015
-
- Zorzi M, Fedeli U, Schievano E, Bovo E, Guzzinati S, Baracco S, et al.Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut 2015;64(5):784-90. - PubMed
References to other published versions of this review
Grobbee 2016
-
- Grobbee EJ.From FIT to Future [PhD thesis]. Rotterdam (NL): Erasmus University Rotterdam, 2016.
Schreuders 2016
-
- Schreuders EH.Optimizing Colorectal Cancer Screening [PhD thesis]. Rotterdam (NL): Erasmus University Rotterdam, 2016.
Van Roon 2011
-
- Van Roon AH, Wilschut JA, Hol L, Van Ballegooijen M, Reijerink JC, 't Mannetje H, et al.Diagnostic yield improves with collection of 2 samples in fecal immunochemical test screening without affecting attendance. Clinical Journal of Gastroenterology and Hepatology 2011;9(4):333-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

