Metabolic reprogramming: a bridge between aging and tumorigenesis
- PMID: 35666002
- PMCID: PMC9490145
- DOI: 10.1002/1878-0261.13261
Metabolic reprogramming: a bridge between aging and tumorigenesis
Abstract
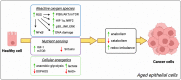
Aging is the most robust risk factor for cancer development, with more than 60% of cancers occurring in those aged 60 and above. However, how aging and tumorigenesis are intertwined is poorly understood and a matter of significant debate. Metabolic changes are hallmarks of both aging and tumorigenesis. The deleterious consequences of aging include dysfunctional cellular processes, the build-up of metabolic byproducts and waste molecules in circulation and within tissues, and stiffer connective tissues that impede blood flow and oxygenation. Collectively, these age-driven changes lead to metabolic reprogramming in different cell types of a given tissue that significantly affects their cellular functions. Here, we put forward the idea that metabolic changes that happen during aging help create a favorable environment for tumorigenesis. We review parallels in metabolic changes that happen during aging and how these changes function both as adaptive mechanisms that enable the development of malignant phenotypes in a cell-autonomous manner and as mechanisms that suppress immune surveillance, collectively creating the perfect environment for cancers to thrive. Hence, antiaging therapeutic strategies that target the metabolic reprogramming that occurs as we age might provide new opportunities to prevent cancer initiation and/or improve responses to standard-of-care anticancer therapies.
Keywords: aging; cellular energetics; immune response; metabolic reprogramming; tumorigenesis.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gensler HL, Bernstein H. DNA damage as the primary cause of aging. Q Rev Biol. 1981;56:279–303. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

