Elevated liver enzymes predict morbidity and mortality despite antiviral cure in patients with chronic hepatitis C: Data from the German Hepatitis C-Registry
- PMID: 35666055
- PMCID: PMC9426389
- DOI: 10.1002/hep4.2015
Elevated liver enzymes predict morbidity and mortality despite antiviral cure in patients with chronic hepatitis C: Data from the German Hepatitis C-Registry
Abstract
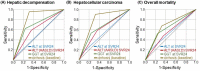
While direct-acting antivirals (DAAs) cure chronic hepatitis C virus (HCV) infection in almost all patients, some patients remain at risk of liver disease despite HCV cure. In order to identify risk factors indicating liver-related morbidity and death after viral cure, we included 6982 patients from the national multicenter real-world German Hepatitis C Registry with regular follow-up visits for up to 7 years after DAA therapy. Definitions for normal liver function tests (in women/men) were alanine aminotransferase (ALT; ≤35/≤50 U/L), ALT according to American Association for the Study of Liver Diseases (AASLD; ≤19/≤30 U/L), and gamma-glutamyltransferase (GGT; ≤40/≤60 U/L). In our cohort, 97.4% of patients achieved sustained virologic response (SVR). At 24 weeks after SVR (SVR24), elevated ALT occurred in 657/6982 (9.4%), elevated ALT (AASLD) in 2609/6982 (37.4%), and elevated GGT in 1777/6982 (25.5%) patients. Risk factors for increased ALT at SVR24 were obesity, alcohol, cirrhosis, elevated baseline ALT, and non-SVR. Increased GGT at SVR24 was significantly (p < 0.05) and independently associated with male sex (odds ratio [OR], 2.12), higher body mass index (OR, 1.04), age >50 years (OR, 1.60), liver cirrhosis (OR, 3.97), alcohol consumption (OR, 2.99), diabetes (OR, 1.63), non-SVR (OR, 8.00), and elevated GGT at baseline (OR, 17.12). In multivariate regression analysis, elevated GGT at SVR24, particularly in combination with cirrhosis, was the best predictor for hepatic decompensation, hepatocellular carcinoma development, and death, followed by elevated ALT (AASLD) and standard ALT, which predicted hepatic decompensation. Despite successful HCV therapy, elevated GGT at SVR24 and to a lesser extent ALT are predictive of the future clinical outcome and linked with liver-associated comorbidities. This may highlight the relevance of nonalcoholic fatty liver disease, diabetes mellitus, alcohol, and cirrhosis for the clinical outcome in a vulnerable population, even after HCV cure.
© 2022 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
Stefan Mauss has received sponsorship for lectures (national or international) from Gilead; he has served on an advisory committee or review panel for Gilead, Janssen, MSD, ViiV. Frank Tacke has received research grants (to the institution) from Allergan, BMS, Gilead, Inventiva; he has been a consultant for Allergan, Bayer, Gilead, BMS, Boehringer, Intercept, Ionis, Inventiva, Merz, Pfizer, Alnylam, NGM, CSL Behring, Novo Nordisk, Novartis; he has received sponsorship for lectures (national or international) from Gilead, AbbVie, Falk, Merz, Intercept. Hartwig Klinker; he has served on an advisory committee or review panel for AbbVie, BMS, Gilead, Janssen, MSD; he has received fees for speaking and teaching from AbbVie, BMS, Gilead, Janssen, MSD. Klaus Boeker has received sponsorship for lectures (national/international) and consultant fees from AbbVie and Gilead. Uta Merle has served on an advisory committee or review panel for CSL‐Behring, Gilead, Takeda; she has received speaking and teaching fees from CSL‐Behring, FALK, MSD. Peter Buggisch has received sponsorship for lectures (national/international) from AbbVie, Gilead, MSD; he has served on an advisory committee or review panel for AbbVie, Gilead, MSD. Dietrich Hüppe has received speaking and teaching fees from AbbVie GmbH, Falk Pharma, Ferring Arzneimittel GmbH. Markus Cornberg has received speaking and teaching fees and has served on an advisory or review panel for Abbvie, Bristol‐Myers Squibb, Gilead Sciences, Janssen‐Cilag, Roche, Merck, MSD, Biogen, Falk Foundation, Boehringer Ingelheim, Siemens, Spring Bank; he has received grants from Roche. Christoph Sarrazin has served on an advisory committee or review panel for AbbVie, Gilead, Merck/MSD; he has received grant/research support from AbbVie, Gilead; he has received speaking and teaching fees from AbbVie, Gilead, Merck/MSD. Heiner Wedemeyer has received sponsorship for lectures (national or international) from, has received grants from, and/or has served as a consultant for Abbott, AbbVie, Altimmune, Biotest, BMS, BTG, Dicerna, Gilead, Janssen, Merck/MSD, MYR GmbH, Novartis, Roche, Siemens, Transgene. Thomas Berg has served as a consultant/advisory board member/investigator/speaker for AbbVie, BMS, Boehringer, Gilead, Janssen, Merck, Novartis, Roche, Vertex Pharmaceuticals; he has received grants from Gilead, Janssen, Novartis, Roche. The other authors have nothing to disclose.
DHC‐R: Peter Schirmacher, Michael P. Manns, Stefan Mauss, Heinz Hartmann, Michael R. Kraus, Pavel Khaykin, Carsten Zamani, Stefan Zeuzem, Maria‐Christina Jung, Christiane Cordes, Willidbold Schiffelholz, Gerd Klausen, Holger Hinrichsen, Axel Baumgarten, Katharina Willuweit, Christoph Antoni, Heribert Knechten, Renate Heyne, Nikolaus Kordecki, Hjordis Möller, Tobias Müller, Michael Priller, Ansgar Rieke, Thomas Lutz, Stefan Christensen, Rainer Günther, Uwe Naumann, Rainer Ullrich, Gerlinde Teuber, Albrecht Stoehr, Christine John, Karl‐Georg Simon.
Figures

References
-
- European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–218. - PubMed
-
- Persico M, Rosato V, Aglitti A, Precone D, Corrado M, De Luna A, et al. Sustained virological response by direct antiviral agents in HCV leads to an early and significant improvement of liver fibrosis. Antivir Ther. 2018;23:129–38. - PubMed
-
- Calvaruso V, Craxi A. Hepatic benefits of HCV cure. J Hepatol. 2020;73:1548–56. - PubMed
-
- Janjua NZ, Wong S, Abdia Y, Jeong D, Buller‐Taylor T, Adu PA, et al. Impact of direct‐acting antivirals for HCV on mortality in a large population‐based cohort study. J Hepatol. 2021;75:1049–57. - PubMed
-
- Negro F. Residual risk of liver disease after hepatitis C virus eradication. J Hepatol. 2021;74:952–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

