N-Acetylcysteine protects against cobalt chloride-induced endothelial dysfunction by enhancing glucose-6-phosphate dehydrogenase activity
- PMID: 35666067
- PMCID: PMC9340863
- DOI: 10.1002/2211-5463.13449
N-Acetylcysteine protects against cobalt chloride-induced endothelial dysfunction by enhancing glucose-6-phosphate dehydrogenase activity
Abstract
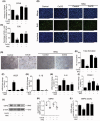
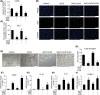
Hypoxia-induced endothelial dysfunction is known to be involved in the pathogenesis of several vascular diseases. However, it remains unclear whether the pentose phosphate pathway (PPP) is involved in regulating the response of endothelial cells to hypoxia. Here, we established an in vitro model by treating EA.hy926 (a hybrid human umbilical vein cell line) with cobalt chloride (CoCl2 ; a chemical mimic that stabilizes HIF-1α, thereby leading to the development of hypoxia), and used this to investigate the involvement of PPP by examining expression of its key enzyme, glucose-6-phosphate dehydrogenase (G6PD). We report that CoCl2 induces the accumulation of HIF-1α, leading to endothelial cell dysfunction characterized by reduced cell viability, proliferation, tube formation, and activation of cytokine production, accompanied with a significant decrease in G6PD expression and activity. The addition of 6-aminonicotinamide (6-AN) to inhibit PPP directly causes endothelial dysfunction. Additionally, N-Acetylcysteine (NAC), a precursor of glutathione, was further evaluated for its protective effects; NAC displayed a protective effect against CoCl2 -induced cell damage by enhancing G6PD activity, and this was abrogated by 6-AN. The effects of CoCl2 and the involvement of G6PD in endothelial dysfunction have been confirmed in primary human aortic endothelial cells. In summary, G6PD was identified as a novel target of CoCl2 -induced damage, which highlighted the involvement of PPP in regulating the response of endothelial cell CoCl2 . Treatment with NAC may be a potential strategy to treat hypoxia or ischemia, which are widely observed in vascular diseases.
Keywords: N-Acetylcysteine; endothelial cells; glucose-6-phosphate dehydrogenase; hypoxia; pentose phosphate pathway.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



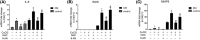
References
-
- Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92:1037–45. - PubMed
-
- Braun L, Kardon T, Reisz‐Porszasz ZS, Banhegyi G, Mandl J. The regulation of the induction of vascular endothelial growth factor at the onset of diabetes in spontaneously diabetic rats. Life Sci. 2001;69:2533–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

