Synergistic Strategies in Aminocatalysis
- PMID: 35666172
- PMCID: PMC9539941
- DOI: 10.1002/chem.202200818
Synergistic Strategies in Aminocatalysis
Abstract
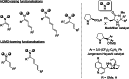
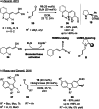
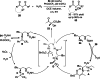
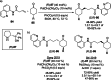
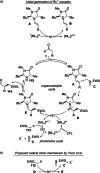
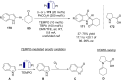
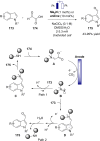
Synergistic catalysis offers the unique possibility of simultaneous activation of both the nucleophile and the electrophile in a reaction. A requirement for this strategy is the stability of the active species towards the reaction conditions and the two concerted catalytic cycles. Since the beginning of the century, aminocatalysis has been established as a platform for the stereoselective activation of carbonyl compounds through HOMO-raising or LUMO-lowering. The burgeoning era of aminocatalysis has been driven by a deep understanding of these activation and stereoinduction modes, thanks to the introduction of versatile and privileged chiral amines. The aim of this review is to cover recent developments in synergistic strategies involving aminocatalysis in combination with organo-, metal-, photo-, and electro-catalysis, focusing on the evolution of privileged aminocatalysts architectures.
Keywords: aminocatalysis; asymmetric catalysis; electrocatalysis; organocatalysis; photoredox catalysis; synergistic catalysis.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















































References
-
- None
-
- List B., Lerner R. A., Barbas C. F., J. Am. Chem. Soc. 2000, 122, 2395–2396;
-
- Ahrendt K. A., Borths C. J., MacMillan D. W. C., J. Am. Chem. Soc. 2000, 122, 4243–4244.
-
- For reviews on asymmetric aminocatalysis see:
-
- Han B., He X.-H., Liu Y.-Q., He G., Peng C., Li J.-L., Chem. Soc. Rev. 2021, 50, 1522–1586; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

