Upfront Modified Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Panitumumab Versus Fluorouracil, Leucovorin, and Oxaliplatin Plus Panitumumab for Patients With RAS/BRAF Wild-Type Metastatic Colorectal Cancer: The Phase III TRIPLETE Study by GONO
- PMID: 35666229
- PMCID: PMC9426812
- DOI: 10.1200/JCO.22.00839
Upfront Modified Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Panitumumab Versus Fluorouracil, Leucovorin, and Oxaliplatin Plus Panitumumab for Patients With RAS/BRAF Wild-Type Metastatic Colorectal Cancer: The Phase III TRIPLETE Study by GONO
Abstract
Purpose: To verify whether the intensification of the upfront chemotherapy backbone with a modified schedule of modified fluorouracil, leucovorin, oxaliplatin, and irinotecan (mFOLFOXIRI) increases the activity of fluorouracil, leucovorin, and oxaliplatin when both regimens are combined with panitumumab as initial treatment for RAS and BRAF wild-type (wt) metastatic colorectal cancer (mCRC).
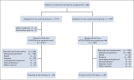
Methods: TRIPLETE was a prospective, open-label, phase III trial in which previously untreated patients with unresectable RAS and BRAF wt mCRC were randomly assigned 1:1 to modified FOLFOX/panitumumab (control group) or mFOLFOXIRI/panitumumab (experimental group) up to 12 cycles, followed by fluorouracil/-leucovorin/panitumumab until disease progression. The primary end point was objective response rate (ORR) according to RECIST 1.1. Hypothesizing an ORR of 60% in the control group, 432 cases provided 90% power to a two-sided chi-square test for heterogeneity with a two-sided alpha error of .05 to detect ≥ 15% differences between arms (ClinicalTrials.gov identifier: NCT03231722).
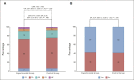
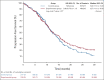
Results: From September 2017 to September 2021, 435 patients were enrolled (control group/experimental group: 217/218) in 57 Italian sites. One hundred sixty (73%) patients treated with mFOLFOXIRI plus panitumumab and 165 (76%) patients treated with modified FOLFOX plus panitumumab achieved RECIST response (odds ratio 0.87, 95% CI, 0.56 to 1.34, P = .526). No differences in early tumor shrinkage rate (57%/58%, P = .878) and deepness of response (median: 48%/47%, P = .845) were reported, nor in R0 resection rate (25%/29%, P = .317). No significant difference between arms was reported in terms of progression-free survival (median progression-free survival: 12.7 in the experimental group v 12.3 months in the control group, hazard ratio: 0.88, 95% CI, 0.70 to 1.11, P = .277).
Conclusion: The intensification of the upfront chemotherapy backbone in combination with panitumumab does not provide additional benefit in terms of treatment activity at the price of increased gastrointestinal toxicity in patients with RAS and BRAF wt mCRC.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures

References
-
- Van Cutsem E, Cervantes A, Adam R, et al. : ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386-1422, 2016 - PubMed
-
- National Comprehensive Cancer Network (NCCN) Guidelines : Colon Cancer Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
-
- Cremolini C, Antoniotti C, Stein A, et al. : Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol 38:3314-3324, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

