Association of Aortic Stiffness and Pressure Pulsatility With Global Amyloid-β and Regional Tau Burden Among Framingham Heart Study Participants Without Dementia
- PMID: 35666520
- PMCID: PMC9171656
- DOI: 10.1001/jamaneurol.2022.1261
Association of Aortic Stiffness and Pressure Pulsatility With Global Amyloid-β and Regional Tau Burden Among Framingham Heart Study Participants Without Dementia
Abstract
Importance: Aortic stiffness is associated with clinical hallmarks of Alzheimer disease and related dementias and could be a modifiable target for disease prevention.
Objective: To assess associations of aortic stiffness and pressure pulsatility with global amyloid-β plaques and regional tau burden in the brain of middle-aged and older adults without dementia.
Design, setting, and participants: The sample for this cross-sectional study was drawn from the Framingham Heart Study Third Generation Cohort at examination 3 (N = 3171; 2016-2019), of whom 3092 successfully underwent comprehensive hemodynamic evaluations. In a supplemental visit (2015-2021), a subset of 270 participants without dementia who represented the spectrum of vascular risk also underwent positron emission tomography. Thirteen participants were excluded for missing covariate data. The final sample size was 257 participants.
Exposures: Three measures of aortic stiffness and pressure pulsatility (carotid-femoral pulse wave velocity, central pulse pressure [CPP], and forward wave amplitude [FWA]) were evaluated using arterial tonometry.
Main outcomes and measures: Global amyloid-β plaques and regional tau were assessed using 11C-Pittsburgh compound B and 18F-flortaucipir positron emission tomography tracers, respectively.
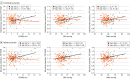
Results: The mean (SD) age of the 257 participants was 54 (8) years, and 126 were women (49%). All participants were White Western European race. In multivariable models, higher CPP (β per SD = 0.17; 95% CI, 0.00-0.35; P = .045) and FWA (β per SD = 0.16; 95% CI, 0.00-0.31; P = .04) were associated with greater entorhinal tau burden. In similar models, higher CPP (β per SD = 0.19; 95% CI, 0.02-0.36; P = .03) and FWA (β per SD = 0.17; 95% CI, 0.01-0.32; P = .03) were associated with greater rhinal tau burden. Aortic stiffness and pressure pulsatility measures were not associated with amygdala, inferior temporal, precuneus tau burden, or global amyloid-β plaques. Associations for entorhinal and rhinal tau outcomes were more prominent in older participants (≥60 years). For example, higher levels of all aortic stiffness and pressure pulsatility measures (β per SD = 0.40-0.92; P = .001-.02) were associated with higher entorhinal tau burden among older but not younger participants in stratified analyses.
Conclusions and relevance: In this cross-sectional study, abnormal central vascular hemodynamics were associated with higher tau burden in specific brain regions. Findings suggest that aortic stiffness, which is potentially modifiable, may be a probable independent target for prevention of tau-related pathologies.
Conflict of interest statement
Figures


References
-
- Nichols W, O’Rourke M, Vlachopoulos C. McDonald’s Blood Flow in Arteries. Theoretical, Experimental and Clinical Principles. 6th ed. Hodder Arnold; 2011.
-
- Gorelick PB, Scuteri A, Black SE, et al. ; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672-2713. doi: 10.1161/STR.0b013e3182299496 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

