Evaluation of Outcomes Among Patients With Traumatic Intracranial Hypertension Treated With Decompressive Craniectomy vs Standard Medical Care at 24 Months: A Secondary Analysis of the RESCUEicp Randomized Clinical Trial
- PMID: 35666526
- PMCID: PMC9171657
- DOI: 10.1001/jamaneurol.2022.1070
Evaluation of Outcomes Among Patients With Traumatic Intracranial Hypertension Treated With Decompressive Craniectomy vs Standard Medical Care at 24 Months: A Secondary Analysis of the RESCUEicp Randomized Clinical Trial
Abstract
Importance: Trials often assess primary outcomes of traumatic brain injury at 6 months. Longer-term data are needed to assess outcomes for patients receiving surgical vs medical treatment for traumatic intracranial hypertension.
Objective: To evaluate 24-month outcomes for patients with traumatic intracranial hypertension treated with decompressive craniectomy or standard medical care.
Design, setting, and participants: Prespecified secondary analysis of the Randomized Evaluation of Surgery With Craniectomy for Uncontrollable Elevation of Intracranial Pressure (RESCUEicp) randomized clinical trial data was performed for patients with traumatic intracranial hypertension (>25 mm Hg) from 52 centers in 20 countries. Enrollment occurred between January 2004 and March 2014. Data were analyzed between 2018 and 2021. Eligibility criteria were age 10 to 65 years, traumatic brain injury (confirmed via computed tomography), intracranial pressure monitoring, and sustained and refractory elevated intracranial pressure for 1 to 12 hours despite pressure-controlling measures. Exclusion criteria were bilateral fixed and dilated pupils, bleeding diathesis, or unsurvivable injury.
Interventions: Patients were randomly assigned 1:1 to receive a decompressive craniectomy with standard care (surgical group) or to ongoing medical treatment with the option to add barbiturate infusion (medical group).
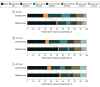
Main outcomes and measures: The primary outcome was measured with the 8-point Extended Glasgow Outcome Scale (1 indicates death and 8 denotes upper good recovery), and the 6- to 24-month outcome trajectory was examined.
Results: This study enrolled 408 patients: 206 in the surgical group and 202 in the medical group. The mean (SD) age was 32.3 (13.2) and 34.8 (13.7) years, respectively, and the study population was predominantly male (165 [81.7%] and 156 [80.0%], respectively). At 24 months, patients in the surgical group had reduced mortality (61 [33.5%] vs 94 [54.0%]; absolute difference, -20.5 [95% CI, -30.8 to -10.2]) and higher rates of vegetative state (absolute difference, 4.3 [95% CI, 0.0 to 8.6]), lower or upper moderate disability (4.7 [-0.9 to 10.3] vs 2.8 [-4.2 to 9.8]), and lower or upper severe disability (2.2 [-5.4 to 9.8] vs 6.5 [1.8 to 11.2]; χ27 = 24.20, P = .001). For every 100 individuals treated surgically, 21 additional patients survived at 24 months; 4 were in a vegetative state, 2 had lower and 7 had upper severe disability, and 5 had lower and 3 had upper moderate disability, respectively. Rates of lower and upper good recovery were similar for the surgical and medical groups (20 [11.0%] vs 19 [10.9%]), and significant differences in net improvement (≥1 grade) were observed between 6 and 24 months (55 [30.0%] vs 25 [14.0%]; χ22 = 13.27, P = .001).
Conclusions and relevance: At 24 months, patients with surgically treated posttraumatic refractory intracranial hypertension had a sustained reduction in mortality and higher rates of vegetative state, severe disability, and moderate disability. Patients in the surgical group were more likely to improve over time vs patients in the medical group.
Trial registration: ISRCTN Identifier: 66202560.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

