Protein-Calixarene Complexation: From Recognition to Assembly
- PMID: 35666543
- PMCID: PMC9350911
- DOI: 10.1021/acs.accounts.2c00206
Protein-Calixarene Complexation: From Recognition to Assembly
Abstract
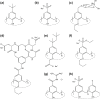
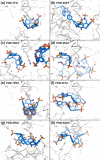
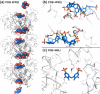
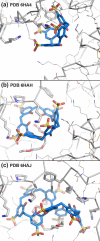
This Account summarizes the progress in protein-calixarene complexation, tracing the developments from binary recognition to the glue activity of calixarenes and beyond to macrocycle-mediated frameworks. During the past 10 years, we have been tackling the question of protein-calixarene complexation in several ways, mainly by cocrystallization and X-ray structure determination as well as by solution state methods, NMR spectroscopy, isothermal titration calorimetry (ITC), and light scattering. Much of this work benefitted from collaboration, highlighted here. Our first breakthrough was the cocrystallization of cationic cytochrome c with sulfonato-calix[4]arene leading to a crystal structure defining three binding sites. Together with NMR studies, a dynamic complexation was deduced in which the calixarene explores the protein surface. Other cationic proteins were similarly amenable to cocrystallization with sulfonato-calix[4]arene, confirming calixarene-arginine/lysine encapsulation and consequent protein assembly. Calixarenes bearing anionic substituents such as sulfonate or phosphonate, but not carboxylate, have proven useful.Studies with larger calix[n]arenes (n = 6, 8) demonstrated the bigger better binder phenomenon with increased affinities and more interesting assemblies, including solution-state oligomerization and porous frameworks. While the calix[4]arene cavity accommodates a single cationic side chain, the larger macrocycles adopt different conformations, molding to the protein surface and accommodating several residues (hydrophobic, polar, and/or charged) in small cavities. In addition to accommodating protein features, the calixarene can bind exogenous components such as polyethylene glycol (PEG), metal ions, buffer, and additives. Ternary cocrystallization of cytochrome c, sulfonato-calix[8]arene, and spermine resulted in altered framework fabrication due to calixarene encapsulation of the tetraamine. Besides host-guest chemistry with exogenous components, the calixarene can also self-assemble, with numerous instances of macrocycle dimers.Calixarene complexation enables protein encapsulation, not merely side chain encapsulation. Cocrystal structures of sulfonato-calix[8]arene with cytochrome c or Ralstonia solanacearum lectin (RSL) provide evidence of encapsulation, with multiple calixarenes masking the same protein. NMR studies of cytochrome c and sulfonato-calix[8]arene are also consistent with multisite binding. In the case of RSL, a C3 symmetric trimer, up to six calixarenes bind the protein yielding a cubic framework mediated by calixarene dimers. Biomolecular calixarene complexation has evolved from molecular recognition to framework construction. This latter development contributes to the challenge in design and preparation of porous molecular materials. Cytochrome c and sulfonato-calix[8]arene form frameworks with >60% solvent in which the degree of porosity depends on the protein:calixarene ratio and the crystallization conditions. Recent developments with RSL led to three frameworks with varying porosity depending on the crystallization conditions, particularly the pH. NMR studies indicate a pH-triggered assembly in which two acidic residues appear to play key roles. The field of supramolecular protein chemistry is growing, and this Account aims to encourage new developments at the interface between biomolecular and synthetic/supramolecular chemistry.
Conflict of interest statement
The author declares no competing financial interest.
Figures


Similar articles
-
Supramolecular Synthons in Protein-Ligand Frameworks.Cryst Growth Des. 2024 Feb 19;24(5):2149-2156. doi: 10.1021/acs.cgd.3c01480. eCollection 2024 Mar 6. Cryst Growth Des. 2024. PMID: 38463617 Free PMC article.
-
Making and Breaking Supramolecular Synthons for Modular Protein Frameworks.Chemistry. 2025 May 19;31(28):e202500732. doi: 10.1002/chem.202500732. Epub 2025 Apr 16. Chemistry. 2025. PMID: 40178192 Free PMC article.
-
Protein-macrocycle polymorphism: crystal form IV of the Ralstonia solanacearum lectin-sulfonato-calix[8]arene complex.Acta Crystallogr D Struct Biol. 2023 Jul 1;79(Pt 7):624-631. doi: 10.1107/S2059798323003832. Epub 2023 Jun 14. Acta Crystallogr D Struct Biol. 2023. PMID: 37314405 Free PMC article.
-
Recognition of lysine residues on protein surfaces using calixarenes and its application.Curr Drug Discov Technol. 2007 Dec;4(4):220-8. doi: 10.2174/157016307783220512. Curr Drug Discov Technol. 2007. PMID: 18045085 Review.
-
Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications.Acc Chem Res. 2014 Jul 15;47(7):1925-34. doi: 10.1021/ar500009g. Epub 2014 Mar 25. Acc Chem Res. 2014. PMID: 24666259 Review.
Cited by
-
How Do Molecular Tweezers Bind to Proteins? Lessons from X-ray Crystallography.Molecules. 2024 Apr 12;29(8):1764. doi: 10.3390/molecules29081764. Molecules. 2024. PMID: 38675584 Free PMC article. Review.
-
Bismacrocycle: Structures and Applications.Molecules. 2023 Aug 13;28(16):6043. doi: 10.3390/molecules28166043. Molecules. 2023. PMID: 37630294 Free PMC article. Review.
-
Design, synthesis, and anti-inflammatory potential of PROTAC drug molecules based on fondaparinux sodium.Front Bioeng Biotechnol. 2025 Jul 7;13:1597344. doi: 10.3389/fbioe.2025.1597344. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40692618 Free PMC article.
-
The Story of the Little Blue Box: A Tribute to Siegfried Hünig.Angew Chem Int Ed Engl. 2023 Jan 2;62(1):e202211387. doi: 10.1002/anie.202211387. Epub 2022 Nov 24. Angew Chem Int Ed Engl. 2023. PMID: 36131604 Free PMC article. Review.
-
The p-diethanolaminomethylcalix[4]arene-incorporated polyacrylonitrile-based facilitated-transport-nanofiber mat for O2/N2 separation.Nanoscale Adv. 2024 May 22;6(14):3573-3581. doi: 10.1039/d4na00019f. eCollection 2024 Jul 9. Nanoscale Adv. 2024. PMID: 38989527 Free PMC article.
References
-
- Gutsche C. D. Calixarenes. Acc. Chem. Res. 1983, 16, 161–170. 10.1021/ar00089a003. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

