A Programmable In Vivo CRISPR Activation Model Elucidates the Oncogenic and Immunosuppressive Functions of MYC in Lung Adenocarcinoma
- PMID: 35666804
- PMCID: PMC9357118
- DOI: 10.1158/0008-5472.CAN-21-4009
A Programmable In Vivo CRISPR Activation Model Elucidates the Oncogenic and Immunosuppressive Functions of MYC in Lung Adenocarcinoma
Abstract
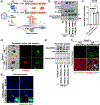
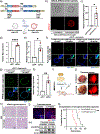
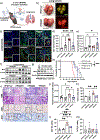
Conventional genetically engineered mouse models (GEMM) are time-consuming, laborious, and offer limited spatiotemporal control. Here, we describe the development of a streamlined platform for in vivo gene activation using CRISPR activation (CRISPRa) technology. Unlike conventional GEMMs, this model system allows for flexible, sustained, and timed activation of one or more target genes using single or pooled lentiviral guides. Myc and Yap1 were used as model oncogenes to demonstrate gene activation in primary pancreatic organoid cultures in vitro and enhanced tumorigenic potential in Myc-activated organoids when transplanted orthotopically in vivo. Implementation of this model as an autochthonous lung cancer model showed that transduction-mediated activation of Myc led to accelerated tumor progression and significantly reduced overall survival relative to nontargeted tumor controls. Furthermore, Myc activation led to the acquisition of an immune suppressive, "cold" tumor microenvironment. Cross-species validation of these results using publicly available RNA/DNA-seq datasets linked MYC to a previously described immunosuppressive molecular subtype in patient tumors, thus identifying a patient cohort that may benefit from combined MYC- and immune-targeted therapies. Overall, this work demonstrates how CRISPRa can be used for rapid functional validation of putative oncogenes and may allow for the identification and evaluation of potential metastatic and oncogenic drivers through competitive screening.
Significance: A streamlined platform for programmable CRISPR gene activation enables rapid evaluation and functional validation of putative oncogenes in vivo.
©2022 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest disclosure statement:
A.M. receives royalties from Cosmos Wisdom Biotechnology and Thrive Earlier Detection, an Exact Sciences Company. A.M. is also a consultant for Freenome and Tezcat Biotechnology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

