Prophylactic tranexamic acid in patients with hematologic malignancy: a placebo-controlled, randomized clinical trial
- PMID: 35667085
- PMCID: PMC9479029
- DOI: 10.1182/blood.2022016308
Prophylactic tranexamic acid in patients with hematologic malignancy: a placebo-controlled, randomized clinical trial
Abstract
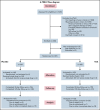
Evidence of the effectiveness of prophylactic use of tranexamic acid (TXA) in thrombocytopenia is lacking. To determine whether TXA safely reduces bleeding incidence in patients undergoing treatment for hematologic malignancies, a randomized, double-blind clinical trial was conducted from June 2016 through June 2020. Of 3120 screened adults, 356 patients were eligible and enrolled, and 337 patients (mean age, 53.9; 141 [41.8%] women), randomized to 1300 mg TXA orally or 1000 mg TXA through IV (n = 168) vs placebo (n = 169) thrice daily for maximum 30 days. Three hundred thirty patients were activated when their platelet counts fell below 30 000 per µL; 279 (83%) had complete outcome ascertainment. World Health Organization (WHO) grade ≥2 bleeding was observed in the 30 days following activation in 50.3% (73/145) and 54.2% (78/144) of patients in the TXA and placebo groups, with an adjusted odds ratio of 0.83 (95% confidence interval [CI], 0.50-1.34; P = .44). There was no statistically significant difference in the mean number of platelet transfusions (mean difference, 0.1; 95% CI, -1.9 to 2.0), mean days alive without grade ≥2 bleeding (mean difference, 0.8; 95% CI, -0.4 to 2.0), thrombotic events (6/163 [3.7%] TXA, 9/163 [5.5%] placebo), or deaths due to serious bleeding. Most common adverse events were: diarrhea (116/164 [70.7%] TXA and 114/163 [69.9%] placebo); febrile neutropenia (111/164 [67.7%] TXA, 105/163 [64.4%] placebo); fatigue (106/164 [64.6%] TXA, 109/163 [66.9%] placebo); and nausea (104/164 [63.4%] TXA, 97/163 [59.5%] placebo). Among patients with hematologic malignancy undergoing chemotherapy or hematopoietic stem cell transplantation, prophylactic treatment with TXA compared with placebo did not significantly reduce the risk of WHO grade ≥2 bleeding.
© 2022 by The American Society of Hematology.
Figures
Comment in
-
Tranexamic acid as a stopgap for low platelets?Blood. 2022 Sep 15;140(11):1185-1186. doi: 10.1182/blood.2022017207. Blood. 2022. PMID: 36107459 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. - PubMed
-
- Han T, Stutzman L, Cohen E, Kim U. Effect of platelet transfusion on hemorrhage in patients with acute leukemia. An autopsy study. Cancer. 1966;19(12):1937-1942. - PubMed
-
- Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB blood collection, utilization, and patient blood management survey. Transfusion. 2016;56(9):2173-2183. - PubMed
-
- Stanworth SJ, Estcourt LJ, Powter G, et al. ; TOPPS Investigators . A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368(19):1771-1780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources