Upregulated SPAG6 promotes acute myeloid leukemia progression through MYO1D that regulates the EGFR family expression
- PMID: 35667090
- PMCID: PMC9631693
- DOI: 10.1182/bloodadvances.2021006920
Upregulated SPAG6 promotes acute myeloid leukemia progression through MYO1D that regulates the EGFR family expression
Abstract
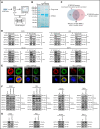
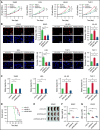
Chromosomal aberrations and gene mutations have been considered to be the major reasons for high recurrence rates and poor survival among acute myeloid leukemia (AML) patients. However, the underlying molecular mechanism of AML gene mutation remains largely unclear. Here, we show that SPAG6 (sperm-associated antigen 6), one of the most markedly increased SPAG genes in AML, significantly contributed to the proliferation and migration of leukemic cells. SPAG6 was highly expressed in AML, and its upregulation was negatively correlated with the prognosis of the disease. In vitro, SPAG6 promoted the proliferation and migration of leukemia cells and promoted cell cycle progression from the G1 phase to the S phase. In vivo, low expression of SPAG6 reduced the proliferation and infiltration of leukemia cells and prolonged the survival of xenograft tumor mice. Furthermore, immunoprecipitation and mass spectrometry analysis showed that SPAG6 interacts with MYO1D (myosin 1D). Specifically, overexpression of SPAG6 promoted the translocation of MYO1D into the cell membrane, thus upgrading the expression level of the EGFR family and thereby promoting the progression of AML. Overall, our study found that SPAG6 combined with MYO1D and translocated MYO1D from the cytosol to the cytomembrane, which induced the PI3K (phosphoinositide 3-kinase)/AKT (protein kinase B) signaling and ERK (extracellular signal-regulated kinase) signaling pathway to regulate the growth and prognosis of AML. SPAG6 may become a new target gene for the treatment of AML.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures







Similar articles
-
Upregulation of SPAG6 in Myelodysplastic Syndrome: Knockdown Inhibits Cell Proliferation via AKT/FOXO Signaling Pathway.DNA Cell Biol. 2019 May;38(5):476-484. doi: 10.1089/dna.2018.4521. Epub 2019 Mar 5. DNA Cell Biol. 2019. PMID: 30835546
-
SPAG6 promotes cell proliferation and inhibits apoptosis through the PTEN/PI3K/AKT pathway in Burkitt lymphoma.Oncol Rep. 2020 Nov;44(5):2021-2030. doi: 10.3892/or.2020.7776. Epub 2020 Sep 22. Oncol Rep. 2020. PMID: 33000212 Free PMC article.
-
SPAG6 silencing induces apoptosis in the myelodysplastic syndrome cell line SKM‑1 via the PTEN/PI3K/AKT signaling pathway in vitro and in vivo.Int J Oncol. 2018 Jul;53(1):297-306. doi: 10.3892/ijo.2018.4390. Epub 2018 May 2. Int J Oncol. 2018. PMID: 29749435
-
Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside.Curr Med Chem. 2007;14(19):2009-23. doi: 10.2174/092986707781368423. Curr Med Chem. 2007. PMID: 17691943 Review.
-
The PI3K-Akt-mTOR Signaling Pathway in Human Acute Myeloid Leukemia (AML) Cells.Int J Mol Sci. 2020 Apr 21;21(8):2907. doi: 10.3390/ijms21082907. Int J Mol Sci. 2020. PMID: 32326335 Free PMC article. Review.
Cited by
-
Diagnostic Algorithm to Subclassify Atypical Spitzoid Tumors in Low and High Risk According to Their Methylation Status.Int J Mol Sci. 2023 Dec 25;25(1):318. doi: 10.3390/ijms25010318. Int J Mol Sci. 2023. PMID: 38203489 Free PMC article.
-
SPAG6 overexpression decreases the pro-apoptotic effect of daunorubicin in acute myeloid leukemia cells through the ROS/JNK MAPK axis in a GSTP1-dependent manner.Front Pharmacol. 2024 Oct 23;15:1390456. doi: 10.3389/fphar.2024.1390456. eCollection 2024. Front Pharmacol. 2024. PMID: 39508041 Free PMC article.
-
Identification and validation of neutrophils-related subtypes and prognosis model in triple negative breast cancer.J Cancer Res Clin Oncol. 2024 Mar 21;150(3):149. doi: 10.1007/s00432-024-05651-3. J Cancer Res Clin Oncol. 2024. PMID: 38512527 Free PMC article.
-
SPAG6 Promotes Multiple Myeloma Through Activation of the MAPK/ERK Signaling Pathway.Front Pharmacol. 2025 Jun 4;16:1572621. doi: 10.3389/fphar.2025.1572621. eCollection 2025. Front Pharmacol. 2025. PMID: 40535772 Free PMC article.
-
CircMYH9 promotes the mRNA stability of SPAG6 by recruiting EIF4A3 to facilitate the progression of breast cancer.Epigenetics. 2025 Dec;20(1):2482382. doi: 10.1080/15592294.2025.2482382. Epub 2025 Mar 27. Epigenetics. 2025. PMID: 40145872 Free PMC article.
References
-
- Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106(4):1154-1163. - PubMed
-
- Heuser M, Ofran Y, Boissel N, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org . Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol 2021;32(6):821]. Ann Oncol. 2020;31(6):697-712. - PubMed
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152. - PubMed
-
- Liersch R, Müller-Tidow C, Berdel WE, Krug U. Prognostic factors for acute myeloid leukaemia in adults – biological significance and clinical use. Br J Haematol. 2014;165(1):17-38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

