Numerical simulations of the nonsymmetric growth and remodeling of arteries under axial twisting
- PMID: 35667148
- PMCID: PMC10782577
- DOI: 10.1016/j.jbiomech.2022.111165
Numerical simulations of the nonsymmetric growth and remodeling of arteries under axial twisting
Abstract
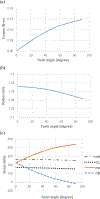
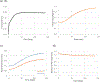
Blood vessels are often subjected to axial twisting during body movement or surgery. Sustained twisting may lead to blood vessel growth and remodeling, however, it remains unclear how the extracellular matrix in the blood vessels remodel under sustained axial twisting. This study aimed to develop a computational model to simulate stress-induced growth and remodeling (G&R) of thin-walled blood vessels under axial twisting. Cylindrical vessels were subjected to a step increase in axial torque while the axial stretch and lumen pressure remained constant. The vessel walls were modeled based on the constrained mixture theory given as microstructure-based discrete fiber families with isotropic matrix structure models. Simulation results demonstrated that in response to a constant twist angle loading, arterial wall thickness, mass, and twisting torque gradually increase towards a new steady state. However, the stress and mass decrease in one diagonal fiber family while increasing in the other diagonal fiber family before reaching plateaus. A novel finding was that the two helical collagen fiber families showed different growth rates and patterns during remodeling, driven by the different fiber stresses generated by the twisting, and led to non-symmetric material properties. This study sheds new light on arterial wall remodeling under axial twisting.
Keywords: Adaptation; Artery; Asymmetric; Fiber model; Mathematical model; Nonsymmetric; Remodeling; Torsion; Twisting.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors have no conflict of interest.
Figures




Similar articles
-
Effects of material non-symmetry on the mechanical behavior of arterial wall.J Mech Behav Biomed Mater. 2022 May;129:105157. doi: 10.1016/j.jmbbm.2022.105157. Epub 2022 Mar 6. J Mech Behav Biomed Mater. 2022. PMID: 35278839
-
The effects of axial twisting and material non-symmetry on arterial bent buckling.J Biomech. 2023 Aug;157:111735. doi: 10.1016/j.jbiomech.2023.111735. Epub 2023 Jul 20. J Biomech. 2023. PMID: 37499429
-
Arterial wall remodeling under sustained axial twisting in rats.J Biomech. 2017 Jul 26;60:124-133. doi: 10.1016/j.jbiomech.2017.06.013. Epub 2017 Jun 21. J Biomech. 2017. PMID: 28693818 Free PMC article.
-
Twist buckling behavior of arteries.Biomech Model Mechanobiol. 2013 Oct;12(5):915-27. doi: 10.1007/s10237-012-0453-0. Epub 2012 Nov 16. Biomech Model Mechanobiol. 2013. PMID: 23160845 Free PMC article.
-
Fundamental role of axial stress in compensatory adaptations by arteries.J Biomech. 2009 Jan 5;42(1):1-8. doi: 10.1016/j.jbiomech.2008.11.011. Epub 2008 Dec 13. J Biomech. 2009. PMID: 19070860 Free PMC article. Review.
References
-
- Baek S, Rajagopal KR and Humphrey JD (2005). “Competition between radial expansion and thickening in the enlargement of an intracranial saccular aneurysm.” Journal of Elasticity 80(1–3): 13–31.
-
- Baek S, Rajagopal KR and Humphrey JD (2006). “A theoretical model of enlarging intracranial fusiform aneurysms.” J Biomech Eng 128(1): 142–9. - PubMed
-
- Bilgin SS, Topalan M, Ip WY and Chow SP (2003). “Effect of torsion on microvenous anastomotic patency in a rat model and early thrombolytic phenomenon.” Microsurgery 23(4): 381–386. - PubMed
-
- Cheng CP, Wilson NM, Hallett RL, Herfkens RJ and Taylor CA (2006). “In vivo MR angiographic quantification of axial and twisting deformations of the superficial femoral artery resulting from maximum hip and knee flexion.” J Vasc Interv Radiol 17(6): 979–987. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

