Bioinspired vaccines to enhance MHC class-I antigen cross-presentation
- PMID: 35667222
- PMCID: PMC9695705
- DOI: 10.1016/j.coi.2022.102215
Bioinspired vaccines to enhance MHC class-I antigen cross-presentation
Abstract
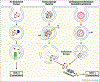
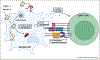
Cross-presentation of exogenous antigen on MHC class-I is a crucial process for generating a CD8+ T cell response, and is therefore an important design consideration in the development of T-cell-engaging vaccines against viruses, intracellular bacteria, and cancers. Here, we briefly summarize known cross-presentation pathways and highlight how synthetic vaccines can be engineered to enhance MHC-I presentation of exogenous peptide and protein antigens by professional antigen-presenting cells (APCs). In particular, we summarize how molecular engineering and nanotechnology are being harnessed to enhance antigen delivery to lymph nodes and to cross-presenting dendritic cells, to bypass endosomal trafficking of exogenous antigen to promote delivery of antigen to the cytosol of APCs, and to coordinate the delivery of antigen with immune-stimulating adjuvants that can act synergistically to augment antigen cross-presentation.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors have no conflicts of interest of direct relevance to the current work. J.T.W. is an inventor on U.S. Patent 10696985 “Reversibly Crosslinked Endosomolytic Polymer Vesicles for Cytosolic Drug Delivery” and on U.S. Patent Application PCT/US2019/058945 “Graft Copolymers, Methods of Forming Graft Copolymers, and Methods of Use Thereof”, which both describe drug-delivery technologies that have been used for delivery of STING agonists as potential vaccine adjuvants.
Figures

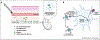
Similar articles
-
Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles.Immunology. 2006 Jan;117(1):78-88. doi: 10.1111/j.1365-2567.2005.02268.x. Immunology. 2006. PMID: 16423043 Free PMC article.
-
Pathways of MHC I cross-presentation of exogenous antigens.Semin Immunol. 2023 Mar;66:101729. doi: 10.1016/j.smim.2023.101729. Epub 2023 Feb 16. Semin Immunol. 2023. PMID: 36804685 Free PMC article. Review.
-
Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages.J Immunol. 2004 May 1;172(9):5277-86. doi: 10.4049/jimmunol.172.9.5277. J Immunol. 2004. PMID: 15100266
-
Mechanisms of antigen presentation to T cells in murine graft-versus-host disease: cross-presentation and the appearance of cross-presentation.Blood. 2011 Dec 8;118(24):6426-37. doi: 10.1182/blood-2011-06-358747. Epub 2011 Sep 30. Blood. 2011. PMID: 21963602 Free PMC article.
-
Regulation of Antigen Export to the Cytosol During Cross-Presentation.Front Immunol. 2019 Jan 28;10:41. doi: 10.3389/fimmu.2019.00041. eCollection 2019. Front Immunol. 2019. PMID: 30745902 Free PMC article. Review.
Cited by
-
Increases in Cellular Immune Responses Due to Positive Effect of CVC1302-Induced Lysosomal Escape in Mice.Vaccines (Basel). 2023 Nov 14;11(11):1718. doi: 10.3390/vaccines11111718. Vaccines (Basel). 2023. PMID: 38006050 Free PMC article.
-
Reinforcing cancer immunotherapy with engineered porous hollow mycobacterium tuberculosis loaded with tumor neoantigens.J Immunother Cancer. 2025 Feb 6;13(2):e010150. doi: 10.1136/jitc-2024-010150. J Immunother Cancer. 2025. PMID: 39915006 Free PMC article.
-
Insect-specific virus platforms for arbovirus vaccine development.Front Immunol. 2025 Mar 14;16:1521104. doi: 10.3389/fimmu.2025.1521104. eCollection 2025. Front Immunol. 2025. PMID: 40160816 Free PMC article. Review.
-
A Cancer Nanovaccine for Co-Delivery of Peptide Neoantigens and Optimized Combinations of STING and TLR4 Agonists.ACS Nano. 2024 Mar 5;18(9):6845-6862. doi: 10.1021/acsnano.3c04471. Epub 2024 Feb 22. ACS Nano. 2024. PMID: 38386282 Free PMC article.
-
Expression and delivery of HA1-M2e antigen using an innovative attenuated Salmonella-mediated delivery system confers promising protection against H9N2 avian influenza challenge.Poult Sci. 2025 Jan;104(1):104602. doi: 10.1016/j.psj.2024.104602. Epub 2024 Nov 27. Poult Sci. 2025. PMID: 39631285 Free PMC article.
References
-
- Wiesel M, Walton S, Richter K, Oxenius A: Virus-specific CD8 T cells: activation, differentiation and memory formation. APMIS 2009, 117:356–381. - PubMed
-
- Dersh D, Holly J, Yewdell JW: A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat Rev Immunol 2021, 21:116–128. - PubMed
-
-
Blass E, Ott PA: Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol 2021, 18:215–229.
This is an excellent review on the development and evaluation of neoantigen-targeted therapeutic cancer vaccines, one of the major emerging applications where vaccines that stimulate robust and durable antitumor CD8+ T cell responses are needed.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

