A subunit vaccine candidate based on the Spike protein of SARS-CoV-2 prevents infectious virus shedding in cats
- PMID: 35667227
- PMCID: PMC9148427
- DOI: 10.1016/j.rvsc.2022.05.003
A subunit vaccine candidate based on the Spike protein of SARS-CoV-2 prevents infectious virus shedding in cats
Abstract
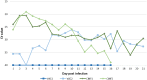
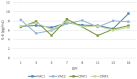
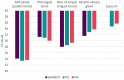
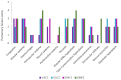
Of the numerous animal species affected by the SARS-CoV-2 virus, cats are one of the most susceptible, and cat-to-cat transmission has been described. Although cat-to-human infection has not, as yet, been demonstrated, preventive measures should be taken in order to avoid both viral infection in cats and transmission among them. In this respect, the application of an effective vaccine to at-risk populations would be a useful tool for controlling the disease in this species. Here, we test a new vaccine prototype based on the Spike protein of the virus in order to prevent infection and infectious virus shedding in cats. The vaccine employed in experimentation, and which is easily produced, triggered a strong neutralizing antibody response in vaccinated animals. In contrast to that which occurred with control animals, no infectious virus was detected in the oropharyngeal or rectal swabs of vaccinated cats submitted to a SARS-CoV-2 challenge. These results are of great interest as regards future considerations related to implementing vaccination programs in pets. The value of cats as vaccination trial models is also described herein.
Keywords: Cats; SARS-CoV-2; Spike protein; Subunit vaccine.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Experimental veterinary SARS-CoV-2 vaccine cross neutralization of the Delta (B.1.617.2) variant virus in cats.Vet Microbiol. 2022 May;268:109395. doi: 10.1016/j.vetmic.2022.109395. Epub 2022 Mar 11. Vet Microbiol. 2022. PMID: 35339817 Free PMC article.
-
Serological Screening for Antibodies against SARS-CoV-2 in Dutch Shelter Cats.Viruses. 2021 Aug 18;13(8):1634. doi: 10.3390/v13081634. Viruses. 2021. PMID: 34452497 Free PMC article.
-
Comprehensive characterization of the antibody responses to SARS-CoV-2 Spike protein finds additional vaccine-induced epitopes beyond those for mild infection.Elife. 2022 Jan 24;11:e73490. doi: 10.7554/eLife.73490. Elife. 2022. PMID: 35072628 Free PMC article.
-
Protective Immunity against SARS Subunit Vaccine Candidates Based on Spike Protein: Lessons for Coronavirus Vaccine Development.J Immunol Res. 2020 Jul 18;2020:7201752. doi: 10.1155/2020/7201752. eCollection 2020. J Immunol Res. 2020. PMID: 32695833 Free PMC article. Review.
-
Identification of B-Cell Epitopes for Eliciting Neutralizing Antibodies against the SARS-CoV-2 Spike Protein through Bioinformatics and Monoclonal Antibody Targeting.Int J Mol Sci. 2022 Apr 14;23(8):4341. doi: 10.3390/ijms23084341. Int J Mol Sci. 2022. PMID: 35457159 Free PMC article. Review.
Cited by
-
Comparative SARS-CoV-2 Omicron BA.5 variant and D614G-Wuhan strain infections in ferrets: insights into attenuation and disease progression during subclinical to mild COVID-19.Front Vet Sci. 2024 Aug 15;11:1435464. doi: 10.3389/fvets.2024.1435464. eCollection 2024. Front Vet Sci. 2024. PMID: 39211479 Free PMC article.
References
-
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. - PMC - PubMed
-
- Barroso-Arévalo S., Barneto A., Ramos Á.M., Rivera B., Sánchez R., Sánchez-Morales L., Pérez-Sancho M., Buendía A., Ferreras E., Ortiz-Menéndez J.C., Moreno I., Serres C., Vela C., Risalde M.Á., Domínguez L., Sánchez-Vizcaíno J.M. Large-scale study on virological and serological prevalence of SARS-CoV-2 in cats and dogs in Spain. Transbound. Emerg. Dis. 2021;1(10):1–16. doi: 10.1111/tbed.14366. - DOI - PMC - PubMed
-
- Barroso-Arévalo S., Sánchez-Morales L., Barasona J.A., Rivera B., Sánchez R., Risalde M.A., Agulló-Ros I., Sánchez-Vizcaíno J.M. Evaluation of the clinical evolution and transmission of SARS-CoV-2 infection in cats by simulating natural routes of infection. Vet. Res. Commun. 2022;3(1–16) doi: 10.1007/s11259-022-09908-5. - DOI - PMC - PubMed
-
- Bosco-Lauth A.M., Hartwig A.E., Porter S.M., Gordy P.W., Nehring M., Byas A.D., VandeWoude S., Ragan I.K., Maison R.M., Bowen R.A. Experimental infection of domestic dogs and cats with SARS-CoV-2: pathogenesis, transmission, and response to reexposure in cats. Proc. Natl. Acad. Sci. 2020;117:26382. - PMC - PubMed
-
- Bukreyev A., Lamirande E.W., Buchholz U.J., Vogel L.N., Elkins W.R., St Claire M., Murphy B.R., Subbarao K., Collins P.L. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet. 2004;363:2122–2127. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

