BRCA1 haploinsufficiency promotes chromosomal amplification under Fenton reaction-based carcinogenesis through ferroptosis-resistance
- PMID: 35667247
- PMCID: PMC9168618
- DOI: 10.1016/j.redox.2022.102356
BRCA1 haploinsufficiency promotes chromosomal amplification under Fenton reaction-based carcinogenesis through ferroptosis-resistance
Abstract
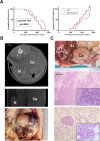
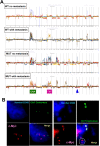
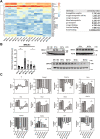
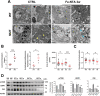
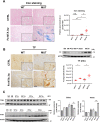
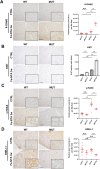
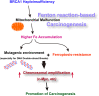
Germline-mutation in BRCA1 tumor suppressor gene is an established risk for carcinogenesis not only in females but also in males. Deficiency in the repair of DNA double-strand breaks is hypothesized as a responsible mechanism for carcinogenesis. However, supporting data is insufficient both in the mutation spectra of cancers in the patients with BRCA1 germline-mutation and in murine knockout/knock-in models of Brca1 haploinsufficiency. Furthermore, information on the driving force toward carcinogenesis in BRCA1 mutation carriers is lacking. Here we applied Fenton reaction-based renal carcinogenesis to a rat heterozygously knockout model of BRCA1 haploinsufficiency (mutant [MUT] model; L63X/+). Rat MUT model revealed significant promotion of renal cell carcinoma (RCC) induced by ferric nitrilotriacetate (Fe-NTA). Array-based comparative genome hybridization of the RCCs identified significant increase in chromosomal amplification, syntenic to those in breast cancers of BRCA1 mutation carriers, including c-Myc, in comparison to those in the wild-type. Subacute-phase analysis of the kidney after repeated Fe-NTA treatment in the MUT model revealed dysregulated iron metabolism with mitochondrial malfunction assessed by expression microarray and electron microscopy, leading to renal tubular proliferation with iron overload. In conclusion, we for the first time demonstrate that biallelic wild-type BRCA1 provides more robust protection for mitochondrial metabolism under iron-catalyzed oxidative stress, preventing the emergence of neoplastic cells with chromosomal amplification. Our results suggest that oxidative stress via excess iron is a major driving force for carcinogenesis in BRCA1 haploinsufficiency, which can be a target for cancer prevention and therapeutics.
Keywords: BRCA1; Chromosomal amplification; Iron; Mitochondria; Renal cell carcinoma.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures
Similar articles
-
BRCA1 haploinsufficiency impairs iron metabolism to promote chrysotile-induced mesothelioma via ferroptosis resistance.Cancer Sci. 2023 Apr;114(4):1423-1436. doi: 10.1111/cas.15705. Epub 2023 Feb 2. Cancer Sci. 2023. PMID: 36541514 Free PMC article.
-
Iron-catalyzed oxidative stress compromises cancer promotional effect of BRCA2 haploinsufficiency through mitochondria-targeted ferroptosis.Redox Biol. 2025 Jun 24;85:103739. doi: 10.1016/j.redox.2025.103739. Online ahead of print. Redox Biol. 2025. PMID: 40609478 Free PMC article.
-
Ferroptosis resistance determines high susceptibility of murine A/J strain to iron-induced renal carcinogenesis.Cancer Sci. 2022 Jan;113(1):65-78. doi: 10.1111/cas.15175. Epub 2021 Nov 23. Cancer Sci. 2022. PMID: 34699654 Free PMC article.
-
Environmental impact on carcinogenesis under BRCA1 haploinsufficiency.Genes Environ. 2023 Jan 13;45(1):2. doi: 10.1186/s41021-023-00258-5. Genes Environ. 2023. PMID: 36639692 Free PMC article. Review.
-
The Role of Ferric Nitrilotriacetate in Renal Carcinogenesis and Cell Death: From Animal Models to Clinical Implications.Cancers (Basel). 2022 Mar 15;14(6):1495. doi: 10.3390/cancers14061495. Cancers (Basel). 2022. PMID: 35326646 Free PMC article. Review.
Cited by
-
Mitochondrial Iron Metabolism: The Crucial Actors in Diseases.Molecules. 2022 Dec 21;28(1):29. doi: 10.3390/molecules28010029. Molecules. 2022. PMID: 36615225 Free PMC article. Review.
-
Brca1L63X /+ rat is a novel model of human BRCA1 deficiency displaying susceptibility to radiation-induced mammary cancer.Cancer Sci. 2022 Oct;113(10):3362-3375. doi: 10.1111/cas.15485. Epub 2022 Aug 21. Cancer Sci. 2022. PMID: 35851737 Free PMC article.
-
Iron as spirit of life to share under monopoly.J Clin Biochem Nutr. 2022 Sep;71(2):78-88. doi: 10.3164/jcbn.22-43. Epub 2022 Jul 27. J Clin Biochem Nutr. 2022. PMID: 36213789 Free PMC article.
-
Knockdown of SLC39A14 inhibits glioma progression by promoting erastin-induced ferroptosis SLC39A14 knockdown inhibits glioma progression.BMC Cancer. 2023 Nov 17;23(1):1120. doi: 10.1186/s12885-023-11637-0. BMC Cancer. 2023. PMID: 37978473 Free PMC article.
-
M6A demethylase FTO-stabilized exosomal circBRCA1 alleviates oxidative stress-induced granulosa cell damage via the miR-642a-5p/FOXO1 axis.J Nanobiotechnology. 2024 Jun 25;22(1):367. doi: 10.1186/s12951-024-02583-5. J Nanobiotechnology. 2024. PMID: 38918838 Free PMC article.
References
-
- Vogelstein B., Kinzler K.W. McGraw-Hill; New York: 1998. The Genetic Basis of Human Cancer.
-
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P.A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L.M., Ding W., et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266(5182):66–71. - PubMed
-
- Liede A., Karlan B.Y., Narod S.A. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J. Clin. Oncol. 2004;22(4):735–742. - PubMed
-
- Narod S.A., Foulkes W.D. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer. 2004;4(9):665–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

