Longitudinal humoral response in MS patients treated with cladribine tablets after receiving the second and third doses of SARS-CoV-2 mRNA vaccine
- PMID: 35667316
- PMCID: PMC9088160
- DOI: 10.1016/j.msard.2022.103863
Longitudinal humoral response in MS patients treated with cladribine tablets after receiving the second and third doses of SARS-CoV-2 mRNA vaccine
Abstract
Background: Multiple sclerosis (MS) patients receive immunomodulatory treatments which can influence their ability to maintain vaccine specific serological response overtime. MS patients treated with cladribine tablets developed a positive serology response following two doses of mRNA COVID-19 vaccine. However, there is only limited data regarding the effect of cladribine tablets on long-term humoral response after the second and the third booster.
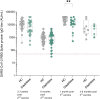
Methods: Serology response to SARS-CoV-2 was tested in healthy controls (HCs) and MS patients treated with cladribine tablets 6 and 9-12 months after the second dose, and 1 and 3-6 months following the third booster-dose of the BTN162b2 mRNA vaccine.
Results: Thirty-five out of 36 MS patients treated with cladribine tablets and 100% (46/46) of HCs had a positive serology response up to 10 months after the second vaccine dose. In addition, all cladribine tablets -treated MS patients (22/22) and HCs (24/24) had a positive robust serology response following the third vaccine with a positive humoral response sustain up to 6 months. One month after the third vaccine dose IgG levels were significantly lower in patients treated with cladribine tablets compared to HCs (15,598+11,313 vs 26,394+11,335, p<0.01). Six-month post second vaccine and 3-6 months post third vaccine there was no difference in IgG levels between the groups (1088.0 ± 1072.0 vs 1153.0 ± 997.1, p = 0.79; 5234+4097 vs 11,198+14,679, p = 0.4).
Conclusion and relevance: MS patients treated with cladribine tablets have sustained positive vaccine specific serology response following the second and third SARS-CoV-2 vaccine dose.
Keywords: COVID-19 vaccination; Cladribine tablets; Long term serology response; Multiple sclerosis.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A. Vaknin-Dembinsky has served on scientific advisory boards for F. Hoffmann-La Roche, Biogen, Sanofi- Aventis and the healthcare business of Merck KGaA, Darmstadt, Germany and; has received grants from F. Hoffmann-La Roche, Biogen and the healthcare business of Merck KGaA, Darmstadt, Germany.
Figures
References
-
- Beutler E. Cladribine (2-chlorodeoxyadenosine) Lancet. 1992;340:952–956. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

