I-SPY COVID adaptive platform trial for COVID-19 acute respiratory failure: rationale, design and operations
- PMID: 35667714
- PMCID: PMC9170797
- DOI: 10.1136/bmjopen-2021-060664
I-SPY COVID adaptive platform trial for COVID-19 acute respiratory failure: rationale, design and operations
Abstract
Introduction: The COVID-19 pandemic brought an urgent need to discover novel effective therapeutics for patients hospitalised with severe COVID-19. The Investigation of Serial studies to Predict Your Therapeutic Response with Imaging And moLecular Analysis (ISPY COVID-19 trial) was designed and implemented in early 2020 to evaluate investigational agents rapidly and simultaneously on a phase 2 adaptive platform. This manuscript outlines the design, rationale, implementation and challenges of the ISPY COVID-19 trial during the first phase of trial activity from April 2020 until December 2021.
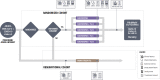
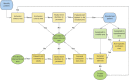
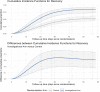
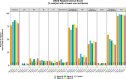
Methods and analysis: The ISPY COVID-19 Trial is a multicentre open-label phase 2 platform trial in the USA designed to evaluate therapeutics that may have a large effect on improving outcomes from severe COVID-19. The ISPY COVID-19 Trial network includes academic and community hospitals with significant geographical diversity across the country. Enrolled patients are randomised to receive one of up to four investigational agents or a control and are evaluated for a family of two primary outcomes-time to recovery and mortality. The statistical design uses a Bayesian model with 'stopping' and 'graduation' criteria designed to efficiently discard ineffective therapies and graduate promising agents for definitive efficacy trials. Each investigational agent arm enrols to a maximum of 125 patients per arm and is compared with concurrent controls. As of December 2021, 11 investigational agent arms had been activated, and 8 arms were complete. Enrolment and adaptation of the trial design are ongoing.
Ethics and dissemination: ISPY COVID-19 operates under a central institutional review board via Wake Forest School of Medicine IRB00066805. Data generated from this trial will be reported in peer-reviewed medical journals.
Trial registration number: NCT04488081.
Keywords: COVID-19; adult intensive & critical care; clinical trials.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DCF has received funding from Quantum Leap Healthcare Collaborative related to this work and from the National Institutes of Health unrelated to this work. DCF has worked as a consultant for Cytovale and Medpace unrelated to this work.
Figures
References
-
- COVID R&D Alliance . Available: https://www.covidrdalliance.com
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
