COVID-19 in Children
- PMID: 35667761
- PMCID: PMC8808705
- DOI: 10.1016/j.pcl.2022.01.013
COVID-19 in Children
Abstract
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus. More than 5 million children have been infected in the United States. Risk factors for more severe disease progression include obesity, pulmonary disease, gastrointestinal disorders, and neurologic comorbidities. Children with COVID-19 are admitted to the pediatric intensive care unit because of severe acute COVID-19 illness or COVID-19-associated multisystem inflammatory syndrome in children. The delta surge of 2021 was responsible for an increased disease burden in children and points to the key role of vaccinating children against this sometimes-deadly disease.
Keywords: ARDS; COVID-19; Epidemiology; MIS-C; Pediatric COVID; Risk factors; SARS-CoV-2.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure The authors have nothing to disclose.
Figures

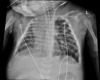


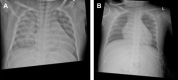
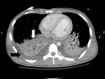


References
-
- Centers for Disease Control and Prevention . 2021. Laboratory-confirmed COVID-19-associated hospitalizations.https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html#virusTypeDiv
-
- Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6):e20200702. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

