Fibroblast Heterogeneity in Healthy and Wounded Skin
- PMID: 35667795
- PMCID: PMC9248828
- DOI: 10.1101/cshperspect.a041238
Fibroblast Heterogeneity in Healthy and Wounded Skin
Abstract
Fibroblasts are the main cell type in the dermis. They are responsible for the synthesis and deposition of structural proteins such as collagen and elastin, which are integrated into the extracellular matrix (ECM). Mouse and human studies using flow cytometry, cell culture, skin reconstitution, and lineage tracing experiments have shown the existence of different subpopulations of fibroblasts, including papillary fibroblasts, reticular fibroblasts, and fibroblasts comprising the dermal papilla at the base of the hair follicle. In recent years, the technological advances in single-cell sequencing have allowed researchers to study the repertoire of cells present in full-thickness skin including the dermis. Multiple groups have confirmed that distinct fibroblast populations can be identified in mouse and human dermis on the basis of differences in the transcriptional profile. Here, we discuss the current state of knowledge regarding dermal fibroblast heterogeneity in healthy mouse and human skin, highlighting the similarities and differences between mouse and human fibroblast subpopulations. We also discuss how fibroblast heterogeneity may provide insights into physiological wound healing and its dysfunction in pathological states such as hypertrophic and keloid scars.
Copyright © 2022 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
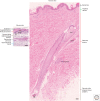


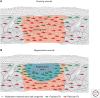
References
LinkOut - more resources
Full Text Sources
