Long-term Treatment With Ponesimod in Relapsing-Remitting Multiple Sclerosis: Results From Randomized Phase 2b Core and Extension Studies
- PMID: 35667837
- PMCID: PMC9484728
- DOI: 10.1212/WNL.0000000000200606
Long-term Treatment With Ponesimod in Relapsing-Remitting Multiple Sclerosis: Results From Randomized Phase 2b Core and Extension Studies
Abstract
Objective: To evaluate the dose-response relationship of 10, 20, and 40 mg ponesimod and long-term efficacy and safety of ponesimod 20 mg using an analysis of combined data from the phase 2 Core and Extension studies in patients with relapsing-remitting multiple sclerosis (RRMS).
Methods: In the Core study, 464 patients were randomized (1:1:1:1): placebo (n = 121), 10 mg (n = 108), 20 mg (n = 116), or 40 mg ponesimod (n = 119) once daily for 24 weeks. Patients who completed the Core study transitioned into the Extension study, which had treatment period 1 (TP1; up to 96 weeks) and TP2 and TP3 (up to 432 weeks). The 40 mg dose was discontinued due to low tolerability at the end of TP1, and the 10 mg dose was subsequently discontinued due to lower benefit-risk profile vs 20 mg at the end of TP2. All patients received 10 or 20 mg during TP2, followed by 20 mg in TP3. Annualized relapse rate (ARR), 6-month confirmed disability accumulation (CDA), time to first confirmed relapse, MRI outcomes, and safety were evaluated.
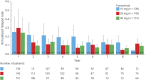
Results: A total of 435 patients received ≥1 dose of ponesimod (first randomized dose: 10 mg = 139, 20 mg = 145, and 40 mg = 151) at any time during the Core and/or the Extension study. As of March 31, 2019, 214 patients were still on ponesimod treatment. The median (range) of ponesimod exposure was 7.95 (0-9.36) years. Ponesimod 20 mg, from Core up to the end of TP3, was associated with sustained low clinical activity (ARR for confirmed relapses: 0.154; at week 432, Kaplan-Meier estimate for confirmed relapse was 43.9%, and 6-month CDA was 20.4%) and MRI disease activity, and over 64% of patients remained free of a confirmed relapse. Most common adverse events were nasopharyngitis (30%), headache (24%), and upper respiratory tract infection (21%).
Conclusion: The effects on multiple sclerosis disease control were maintained with ponesimod 20 mg for approximately 8 years with no new safety concerns identified.
Classification of evidence: This study provides Class IV evidence that in individuals with RRMS, long-term treatment with ponesimod 20 mg was associated with a sustained low annualized confirmed relapse rate (0.154 at week 432), with 64% of patients remaining relapse-free.
Trial registration information: EudraCT Number 2008-006786-92 (Core study) and EudraCT Number 2009-011470-15 (Extension study).
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
-
- Dobson R, Giovannoni G. Multiple sclerosis–a review. Eur J Neurol. 2019;26(1):27-40. - PubMed
-
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622-1636. - PubMed
-
- Degenhardt A, Ramagopalan SV, Scalfari A, Ebers GC. Clinical prognostic factors in multiple sclerosis: a natural history review. Nat Rev Neurol. 2009;5(12):672-682. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
