Cross-sectional seroprevalence surveys of SARS-CoV-2 antibodies in children in Germany, June 2020 to May 2021
- PMID: 35668073
- PMCID: PMC9170697
- DOI: 10.1038/s41467-022-30482-6
Cross-sectional seroprevalence surveys of SARS-CoV-2 antibodies in children in Germany, June 2020 to May 2021
Abstract
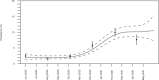
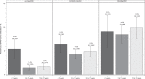
The rate of SARS-CoV-2 infections in children remains unclear due to many asymptomatic cases. We present a study of cross-sectional seroprevalence surveys of anti-SARS-CoV-2 IgG in 10,358 children recruited in paediatric hospitals across Germany from June 2020 to May 2021. Seropositivity increased from 2.0% (95% CI 1.6, 2.5) to 10.8% (95% CI 8.7, 12.9) in March 2021 with little change up to May 2021. Rates increased by migrant background (2.8%, 4.4% and 7.8% for no, one and two parents born outside Germany). Children under three were initially 3.6 (95% CI 2.3, 5.7) times more likely to be seropositive with levels equalising later. The ratio of seropositive cases per recalled infection decreased from 8.6 to 2.8. Since seropositivity exceeds the rate of recalled infections considerably, serologic testing may provide a more valid estimate of infections, which is required to assess both the spread and the risk for severe outcomes of SARS-CoV-2 infections.
© 2022. The Author(s).
Conflict of interest statement
The study centres received academic research funding from the Federal Ministry of Education and Research (BMBF) for study planning, study management and reimbursement for the assay of this study. V.C. reported a patent on methods and reagents for diagnosis of SARS-CoV-2 infection (Pub number 20210190797) of Charité and Euroimmun GmbH.H.v.B. reported receiving honoraria for ongoing reports on the safety profile of an IgG-product from Octapharma GmBH, honoraria for lectures from CSL Behring, honoraria for advisory boards from Takeda and Swedish Orphan Biovitrum AB (publ) (SobiTM) and the ‘treatment guideline for Primary ‘immunodeficiencies’ is under his unpaid leadership. L.N. reported institutional fees for study participation by the German Centre for Lung Research, Vertex Pharmaceuticals and Boehringer Ingelheim. He participates on Trial Steering Committee for CF STORM and is the medical leader of the German CF-registry, and the pharmacovigilance study manager of the ECFSPR.G.H. reported receiving consulting fees from Sanofi GmbH and honoraria for lectures from MedUpdate and Abbvie.No other disclosures were reported.
Figures
References
-
- Armann, J. et al. Risk factors for hospitalization, disease severity and mortality in children and adolescents with COVID-19: Results from a nationwide German registry. Preprint at https://www.medrxiv.org/content/early/2021/06/13/2021.06.07.21258488 (2021).
-
- Viner, R. M. et al. Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch Dis Child, 10.1136/archdischild-2020-320972 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

