Syntaxin 18 regulates the DNA damage response and epithelial-to-mesenchymal transition to promote radiation resistance of lung cancer
- PMID: 35668077
- PMCID: PMC9170725
- DOI: 10.1038/s41419-022-04978-4
Syntaxin 18 regulates the DNA damage response and epithelial-to-mesenchymal transition to promote radiation resistance of lung cancer
Abstract
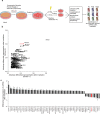
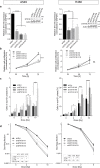
Radiotherapy is an important modality in lung cancer treatment. Despite advances in treatment planning and dose delivery, patient benefit is still limited by in-field relapse and metastatic recurrence. Simultaneous application of cisplatinum-based chemotherapy leads to moderately improved outcomes, thus providing proof-of-concept for radiosensitization strategies in lung cancer. In an unbiased functional genetic screen for radiosensitization targets in lung cancer, we identified syntaxin 18, a protein involved in retrograde vesicular transport between the Golgi apparatus and endoplasmic reticulum, as mediator of radioresistance. Downregulation of endogenous syntaxin 18 specifically reduced clonogenic survival of radioresistant and radiosensitive lung cancer cells following X-radiation. Gene expression programs regulating DNA repair, mitotic checkpoints and mitosis were altered in isogenic cells with reduced syntaxin 18 expression. Functionally, this translated into impaired DNA damage-induced cell cycle checkpoints leading to cell death by mitotic catastrophe. Interestingly, downregulation of syntaxin 18 in lung cancer cells also impaired expression of markers of epithelial-mesenchymal-transition, and reduced migration and invasion capacity. These findings suggest that syntaxin 18 is a key player regulating genes responsible for controlling the growth of the primary tumor as well as metastases upon radiotherapy of lung cancer. They provide a promising lead for biologically rational radiosensitization strategies impacting on radiation-induced cell death as well as metastasis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

