Thinking outside the box: non-canonical targets in multiple sclerosis
- PMID: 35668103
- PMCID: PMC9169033
- DOI: 10.1038/s41573-022-00477-5
Thinking outside the box: non-canonical targets in multiple sclerosis
Abstract
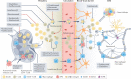
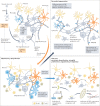
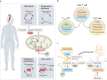
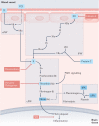
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system that causes demyelination, axonal degeneration and astrogliosis, resulting in progressive neurological disability. Fuelled by an evolving understanding of MS immunopathogenesis, the range of available immunotherapies for clinical use has expanded over the past two decades. However, MS remains an incurable disease and even targeted immunotherapies often fail to control insidious disease progression, indicating the need for new and exceptional therapeutic options beyond the established immunological landscape. In this Review, we highlight such non-canonical targets in preclinical MS research with a focus on five highly promising areas: oligodendrocytes; the blood-brain barrier; metabolites and cellular metabolism; the coagulation system; and tolerance induction. Recent findings in these areas may guide the field towards novel targets for future therapeutic approaches in MS.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
New Insights into Multiple Sclerosis Mechanisms: Lipids on the Track to Control Inflammation and Neurodegeneration.Int J Mol Sci. 2021 Jul 7;22(14):7319. doi: 10.3390/ijms22147319. Int J Mol Sci. 2021. PMID: 34298940 Free PMC article. Review.
-
Chronic Demyelination and Axonal Degeneration in Multiple Sclerosis: Pathogenesis and Therapeutic Implications.Curr Neurol Neurosci Rep. 2021 Apr 9;21(6):26. doi: 10.1007/s11910-021-01110-5. Curr Neurol Neurosci Rep. 2021. PMID: 33835275 Review.
-
Axonal degeneration in multiple sclerosis: can we predict and prevent permanent disability?Acta Neuropathol Commun. 2014 Aug 27;2:97. doi: 10.1186/s40478-014-0097-7. Acta Neuropathol Commun. 2014. PMID: 25159125 Free PMC article. Review.
-
Multiple sclerosis: a review of existing therapy and future prospects.J Pak Med Assoc. 1996 Jan;46(1):20-4. J Pak Med Assoc. 1996. PMID: 8830165 Review.
-
Emerging immunopharmacological targets in multiple sclerosis.J Neurol Sci. 2015 Nov 15;358(1-2):22-30. doi: 10.1016/j.jns.2015.09.346. Epub 2015 Sep 14. J Neurol Sci. 2015. PMID: 26440421 Free PMC article. Review.
Cited by
-
The neuroimmune axis of Alzheimer's disease.Genome Med. 2023 Jan 26;15(1):6. doi: 10.1186/s13073-023-01155-w. Genome Med. 2023. PMID: 36703235 Free PMC article. Review.
-
Mechanisms underlying the beneficial effects of physical exercise on multiple sclerosis: focus on immune cells.Front Immunol. 2023 Sep 29;14:1260663. doi: 10.3389/fimmu.2023.1260663. eCollection 2023. Front Immunol. 2023. PMID: 37841264 Free PMC article. Review.
-
GSDME-mediated pyroptosis in microglia exacerbates demyelination and neuroinflammation in multiple sclerosis: insights from humans and cuprizone-induced demyelination model mice.Cell Death Differ. 2025 Jun 25. doi: 10.1038/s41418-025-01537-0. Online ahead of print. Cell Death Differ. 2025. PMID: 40555745
-
Glia Connect Inflammation and Neurodegeneration in Multiple Sclerosis.Neurosci Bull. 2023 Mar;39(3):466-478. doi: 10.1007/s12264-023-01034-9. Epub 2023 Feb 28. Neurosci Bull. 2023. PMID: 36853544 Free PMC article. Review.
-
Animal models to investigate the effects of inflammation on remyelination in multiple sclerosis.Front Mol Neurosci. 2022 Nov 3;15:995477. doi: 10.3389/fnmol.2022.995477. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36407761 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

