Omicron-specific mRNA vaccination alone and as a heterologous booster against SARS-CoV-2
- PMID: 35668119
- PMCID: PMC9169595
- DOI: 10.1038/s41467-022-30878-4
Omicron-specific mRNA vaccination alone and as a heterologous booster against SARS-CoV-2
Abstract
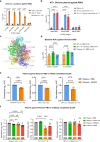
The Omicron variant of SARS-CoV-2 recently swept the globe and showed high level of immune evasion. Here, we generate an Omicron-specific lipid nanoparticle (LNP) mRNA vaccine candidate, and test its activity in animals, both alone and as a heterologous booster to WT mRNA vaccine. Our Omicron-specific LNP-mRNA vaccine elicits strong antibody response in vaccination-naïve mice. Mice that received two-dose WT LNP-mRNA show a > 40-fold reduction in neutralization potency against Omicron than WT two weeks post boost, which further reduce to background level after 3 months. The WT or Omicron LNP-mRNA booster increases the waning antibody response of WT LNP-mRNA vaccinated mice against Omicron by 40 fold at two weeks post injection. Interestingly, the heterologous Omicron booster elicits neutralizing titers 10-20 fold higher than the homologous WT booster against Omicron variant, with comparable titers against Delta variant. All three types of vaccination, including Omicron alone, WT booster and Omicron booster, elicit broad binding antibody responses against SARS-CoV-2 WA-1, Beta, Delta variants and SARS-CoV. These data provide direct assessments of an Omicron-specific mRNA vaccination in vivo, both alone and as a heterologous booster to WT mRNA vaccine.
© 2022. The Author(s).
Conflict of interest statement
A patent application has been filed by Yale University related to the data described here (inventors: S.C., L.P., Z.F., and P.R.). Yale University has committed to rapidly executable nonexclusive royalty-free licenses to intellectual property rights for the purpose of making and distributing products to prevent, diagnose, and treat COVID-19 infection during the pandemic and for a short period thereafter. S.C. is a scientific Founder of EvolveImmune Tx and Cellinfinity Bio, unrelated to this study. The remaining authors declare no competing interests.
Figures



Update of
-
Omicron-specific mRNA vaccination alone and as a heterologous booster against SARS-CoV-2.bioRxiv [Preprint]. 2022 Feb 28:2022.02.14.480449. doi: 10.1101/2022.02.14.480449. bioRxiv. 2022. Update in: Nat Commun. 2022 Jun 6;13(1):3250. doi: 10.1038/s41467-022-30878-4. PMID: 35194606 Free PMC article. Updated. Preprint.
References
-
- Centers for Disease Control and Prevention. Science Brief: Omicron (B.1.1.529) Variant (2021). - PubMed
-
- UK Health Security Agency. Omicron daily overview: 31 December 2021. (2021).
-
- Pulliam, J. et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. MedRxiv10.1101/2021.11.11.21266068 (2021).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

