Inhibition of type 1 immunity with tofacitinib is associated with marked improvement in longstanding sarcoidosis
- PMID: 35668129
- PMCID: PMC9170782
- DOI: 10.1038/s41467-022-30615-x
Inhibition of type 1 immunity with tofacitinib is associated with marked improvement in longstanding sarcoidosis
Abstract
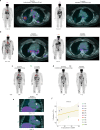
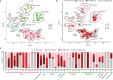
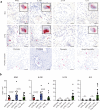
Sarcoidosis is an idiopathic inflammatory disorder that is commonly treated with glucocorticoids. An imprecise understanding of the immunologic changes underlying sarcoidosis has limited therapeutic progress. Here in this open-label trial (NCT03910543), 10 patients with cutaneous sarcoidosis are treated with tofacitinib, a Janus kinase inhibitor. The primary outcome is the change in the cutaneous sarcoidosis activity and morphology instrument (CSAMI) activity score after 6 months of treatment. Secondary outcomes included change in internal organ involvement, molecular parameters, and safety. All patients experience improvement in their skin with 6 patients showing a complete response. Improvement in internal organ involvement is also observed. CD4+ T cell-derived IFN-γ is identified as a central cytokine mediator of macrophage activation in sarcoidosis. Additional type 1 cytokines produced by distinct cell types, including IL-6, IL-12, IL-15 and GM-CSF, also associate with pathogenesis. Suppression of the activity of these cytokines, especially IFN-γ, correlates with clinical improvement. Our results thus show that tofacitinib treatment is associated with improved sarcoidosis symptoms, and predominantly acts by inhibiting type 1 immunity.
© 2022. The Author(s).
Conflict of interest statement
W.D. has research funding from Pfizer and Advanced Cell Diagnostics/Bio-techne, serves as a consultant for Eli Lilly, Pfizer, Incyte, and Twi Biotechnology, and receives licensing fees from EMD/Millipore/Sigma. B.D.Y receives research funding from Pfizer. E.J.M. receives research funding from Alnylam, Pfizer, and Eidos and serves as a consultant for Alnylam, Pfizer, and Eidos. D.P. receives consulting fees from Telix Pharmaceuticals and Cohere Health. M.B. is a consultant for Eli Lilly and receives licensing fees from EMD/Millipore//Sigma. R.A.F. is a consultant for Glaxo Smith Kline and Zai labs. B.K. is a consultant to and/or has served on advisory boards for Aclaris Therapeutics, Arena Pharmaceuticals, Bristol-Meyers Squibb, Concert Pharmaceuticals Inc, Dermavant Sciences, Eli Lilly and Company, Pfizer, and VielaBio; he is on speaker’s bureau for Pfizer, Regeneron and Sanofi Genzyme. A.W., D.J.K., K.S., M.J.M., J.D., A.C., R.A., C.R., M.K.M., I.D.O., R.F.C., R.H. and M.G. have no disclosures.
Figures






Comment in
-
Sarcoidosis: can tofacitinib slay the dragon?Nat Rev Rheumatol. 2022 Oct;18(10):557-558. doi: 10.1038/s41584-022-00832-1. Nat Rev Rheumatol. 2022. PMID: 35999390 No abstract available.
-
Tofacitinib for sarcoidosis, a new potential treatment.Int J Rheum Dis. 2022 Nov;25(11):1217-1219. doi: 10.1111/1756-185X.14441. Int J Rheum Dis. 2022. PMID: 36320145 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

