P2RY2-AKT activation is a therapeutically actionable consequence of XPO1 inhibition in acute myeloid leukemia
- PMID: 35668193
- PMCID: PMC9949365
- DOI: 10.1038/s43018-022-00394-x
P2RY2-AKT activation is a therapeutically actionable consequence of XPO1 inhibition in acute myeloid leukemia
Abstract
Selinexor is a first-in-class inhibitor of the nuclear exportin XPO1 that was recently approved by the US Food and Drug Administration for the treatment of multiple myeloma and diffuse large B-cell lymphoma. In relapsed/refractory acute myeloid leukemia (AML), selinexor has shown promising activity, suggesting that selinexor-based combination therapies may have clinical potential. Here, motivated by the hypothesis that selinexor's nuclear sequestration of diverse substrates imposes pleiotropic fitness effects on AML cells, we systematically catalog the pro- and anti-fitness consequences of selinexor treatment. We discover that selinexor activates PI3Kγ-dependent AKT signaling in AML by upregulating the purinergic receptor P2RY2. Inhibiting this axis potentiates the anti-leukemic effects of selinexor in AML cell lines, patient-derived primary cultures and multiple mouse models of AML. In a syngeneic, MLL-AF9-driven mouse model of AML, treatment with selinexor and ipatasertib outperforms both standard-of-care chemotherapy and chemotherapy with selinexor. Together, these findings establish drug-induced P2RY2-AKT signaling as an actionable consequence of XPO1 inhibition in AML.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures

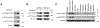


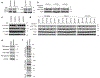


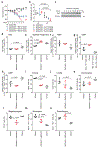



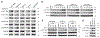




Comment in
-
AKTing on XPO1 inhibition in AML.Nat Cancer. 2022 Jul;3(7):787-789. doi: 10.1038/s43018-022-00395-w. Nat Cancer. 2022. PMID: 35882999 No abstract available.
References
-
- Chari A et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N Engl J Med 381, 727–738 (2019). - PubMed
-
- Kalakonda N et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 7, e511–e522 (2020). - PubMed
-
- Mahipal A, Malafa M, Importins and exportins as therapeutic targets in cancer. Pharmacol Ther 164, 135–143 (2016). - PubMed
-
- Senapedis WT, Baloglu E, Landesman Y, Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol 27, 74–86 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

