The circadian clock protein Rev-erbα provides neuroprotection and attenuates neuroinflammation against Parkinson's disease via the microglial NLRP3 inflammasome
- PMID: 35668454
- PMCID: PMC9169406
- DOI: 10.1186/s12974-022-02494-y
The circadian clock protein Rev-erbα provides neuroprotection and attenuates neuroinflammation against Parkinson's disease via the microglial NLRP3 inflammasome
Abstract
Background: Circadian disturbance is a common nonmotor complaint in Parkinson's disease (PD). The molecular basis underlying circadian rhythm in PD is poorly understood. Neuroinflammation has been identified as a key contributor to PD pathology. In this study, we explored the potential link between the core clock molecule Rev-erbα and the microglia-mediated NLR family pyrin domain-containing 3 (NLRP3) inflammasome in PD pathogenesis.
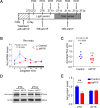
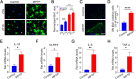
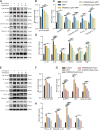
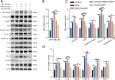
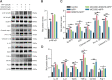
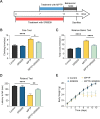
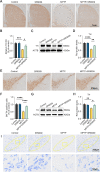
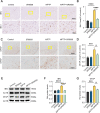
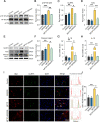
Methods: We first examined the diurnal Rev-erbα rhythms and diurnal changes in microglia-mediated inflammatory cytokines expression in the SN of MPTP-induced PD mice. Further, we used BV2 cell to investigate the impacts of Rev-erbα on NLRP3 inflammasome and microglial polarization induced by 1-methyl-4-phenylpyridinium (MPP+) and αsyn pre-formed fibril. The role of Rev-erbα in regulating microglial activation via NF-κB and NLRP3 inflammasome pathway was then explored. Effects of SR9009 against NLRP3 inflammasome activation, microgliosis and nigrostriatal dopaminergic degeneration in the SN and striatum of MPTP-induced PD mice were studied in detail.
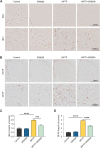
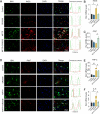
Results: BV2 cell-based experiments revealed the role of Rev-erbα in regulating microglial activation and polarization through the NF-κB and NLRP3 inflammasome pathways. Circadian oscillation of the core clock gene Rev-erbα in the substantia nigra (SN) disappeared in MPTP-induced PD mice, as well as diurnal changes in microglial morphology. The expression of inflammatory cytokines in SN of the MPTP-induced mice were significantly elevated. Furthermore, dopaminergic neurons loss in the nigrostriatal system were partially reversed by SR9009, a selective Rev-erbα agonist. In addition, SR9009 effectively reduced the MPTP-induced glial activation, microglial polarization and NLRP3 inflammasome activation in the nigrostriatal system.
Conclusions: These observations suggest that the circadian clock protein Rev-erbα plays an essential role in attenuating neuroinflammation in PD pathology, and provides a potential therapeutic target for PD treatment.
Keywords: Circadian rhythm; NLRP3; Neuroinflammation; Parkinson’s disease; Rev-erbα.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration.Cell Death Differ. 2019 Jan;26(2):213-228. doi: 10.1038/s41418-018-0124-5. Epub 2018 May 21. Cell Death Differ. 2019. PMID: 29786072 Free PMC article.
-
TREM2 Deficiency Aggravates NLRP3 Inflammasome Activation and Pyroptosis in MPTP-Induced Parkinson's Disease Mice and LPS-Induced BV2 Cells.Mol Neurobiol. 2024 May;61(5):2590-2605. doi: 10.1007/s12035-023-03713-0. Epub 2023 Nov 2. Mol Neurobiol. 2024. PMID: 37917301 Free PMC article.
-
RND3 modulates microglial polarization and alleviates neuroinflammation in Parkinson's disease by suppressing NLRP3 inflammasome activation.Exp Cell Res. 2024 Jun 1;439(1):114088. doi: 10.1016/j.yexcr.2024.114088. Epub 2024 May 12. Exp Cell Res. 2024. PMID: 38744409
-
Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson's Disease.Front Immunol. 2021 Oct 8;12:719807. doi: 10.3389/fimmu.2021.719807. eCollection 2021. Front Immunol. 2021. PMID: 34691027 Free PMC article. Review.
-
NF-κB/NLRP3 inflammasome axis and risk of Parkinson's disease in Type 2 diabetes mellitus: A narrative review and new perspective.J Cell Mol Med. 2023 Jul;27(13):1775-1789. doi: 10.1111/jcmm.17784. Epub 2023 May 21. J Cell Mol Med. 2023. PMID: 37210624 Free PMC article. Review.
Cited by
-
Epigenetic events influencing the biological clock: Panacea for neurodegeneration.Heliyon. 2024 Oct 2;10(19):e38836. doi: 10.1016/j.heliyon.2024.e38836. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39430507 Free PMC article. Review.
-
Circadian Clock Disruption and Non-type 2 Asthma: A Hypothesis-Driven Perspective on Immune, Epithelial, and Steroid Response.Clin Rev Allergy Immunol. 2025 Jul 25;68(1):72. doi: 10.1007/s12016-025-09088-5. Clin Rev Allergy Immunol. 2025. PMID: 40707819 Review.
-
Inflammasomes in neurological disorders - mechanisms and therapeutic potential.Nat Rev Neurol. 2024 Feb;20(2):67-83. doi: 10.1038/s41582-023-00915-x. Epub 2024 Jan 9. Nat Rev Neurol. 2024. PMID: 38195712 Review.
-
REV-ERBα Mitigates Astrocyte Activation and Protects Dopaminergic Neurons from Damage.J Mol Neurosci. 2024 Sep 10;74(3):84. doi: 10.1007/s12031-024-02264-w. J Mol Neurosci. 2024. PMID: 39254874
-
New insight on microglia activation in neurodegenerative diseases and therapeutics.Front Neurosci. 2023 Dec 22;17:1308345. doi: 10.3389/fnins.2023.1308345. eCollection 2023. Front Neurosci. 2023. PMID: 38188026 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 81671260/National Natural Science Foundation of China
- 81974201/National Natural Science Foundation of China
- 2017YFC1310200/National Key Plan for Scientific Research and Development of China
- 2016YFC1306000/National Key Plan for Scientific Research and Development of China
- 2019HSA02/Chinese Sleep Research Society Hansoh Project
LinkOut - more resources
Full Text Sources
Medical

