Ruxolitinib-corticosteroid as first-line therapy for newly diagnosed high-risk acute graft versus host disease: study protocol for a multicenter, randomized, phase II controlled trial
- PMID: 35668528
- PMCID: PMC9169300
- DOI: 10.1186/s13063-022-06426-2
Ruxolitinib-corticosteroid as first-line therapy for newly diagnosed high-risk acute graft versus host disease: study protocol for a multicenter, randomized, phase II controlled trial
Abstract
Background: The response rate of the first-line therapy with corticosteroid for acute graft versus host disease (aGVHD) is about 50%, and steroid-refractory disease is associated with high mortality. The improved response rate to the first-line therapy of newly diagnosed aGVHD patients would result in therapeutic benefits. Ruxolitinib, a selective Janus kinase (JAK) 1/2 inhibitor, has been approved for the treatment of steroid-refractory acute GVHD. The addition of ruxolitinib to the first-line therapy may improve the efficacy of corticosteroids.
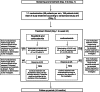
Methods: This investigator-initiated, open-label, multicenter, prospective randomized, and controlled two-arm phase II study compares the efficacy and safety of ruxolitinib combined with 1 mg/kg methylprednisolone versus 2 mg/kg methylprednisolone alone in newly diagnosed aGVHD patients. Patients with intermediate or high-risk aGVHD, as defined by the Minnesota aGVHD high-risk score and biomarker algorithm, are eligible for this study. A total of 198 patients will be randomized at a 1:1 ratio and assigned a GVHD risk (intermediate versus high risk) and disease status before transplantation (complete remission versus no complete remission). The primary endpoint is the overall response rate on day 28, which is defined as an improvement of at least one stage in the severity of aGVHD in one organ without deterioration in any other organ or disappearance of any GVHD signs from all organs without requiring new systemic immunosuppressive treatment. The secondary objectives consist of response time, response duration, overall survival, disease-free survival, non-relapse mortality, failure-free survival, and changes in serum levels of proinflammatory cytokines and GVHD-related biomarkers.
Discussion: This open-label, multicenter, two-arm randomized trial will evaluate whether the addition of ruxolitinib combined with corticosteroid is superior to corticosteroid alone in newly diagnosed high-risk aGVHD.
Trial registration: ClinicalTrials.gov NCT04061876 (version number: 2019.5.18). Registered on July 16, 2019.
Keywords: Acute graft versus host disease; Biomarker; First-line therapy; Ruxolitinib.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Dou L, Hou C, Ma C, Li F, Gao X, Huang W, Wang S, Gao C, Yu L, Liu D. Reduced risk of chronic GVHD by low-dose rATG in adult matched sibling donor peripheral blood stem cell transplantation for hematologic malignancies. Ann Hematol. 2020;99(1):167–179. doi: 10.1007/s00277-019-03884-8. - DOI - PMC - PubMed
-
- Dou LP, Li HH, Wang L, Li F, Huang WR, Yu L, Liu DH. Efficacy and safety of unmanipulated haploidentical related donor allogeneic peripheral blood stem cell transplantation in patients with relapsed/refractory acute myeloid leukemia. Chin Med J. 2018;131(7):790–798. doi: 10.4103/0366-6999.228243. - DOI - PMC - PubMed
-
- Hou C, Dou L, Jia M, Li F, Wang S, Gao X, et al. Ruxolitinib Combined with Corticosteroids as First-Line Therapy for Acute Graft-versus-Host Disease in Haploidentical Peripheral Blood Stem Cell Transplantation Recipients. Transplant Cell Ther. 202;27(1):75.e1–75.e10. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials