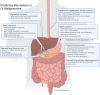
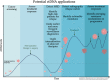
The current state of molecular profiling in gastrointestinal malignancies
- PMID: 35668531
- PMCID: PMC9172079
- DOI: 10.1186/s13062-022-00322-0
The current state of molecular profiling in gastrointestinal malignancies
Abstract
This is a review of the current state of molecular profiling in gastrointestinal (GI) cancers and what to expect from this evolving field in the future. Individualized medicine is moving from broad panel testing of numerous genes or gene products in tumor biopsy samples, identifying biomarkers of prognosis and treatment response, to relatively noninvasive liquid biopsy assays, building on what we have learned in our tumor analysis and growing into its own evolving predictive and prognostic subspecialty. Hence, the field of GI precision oncology is exploding, and this review endeavors to summarize where we are now in preparation for the journey ahead.
Keywords: Biomarker; CTC; Colorectal cancer; Gastroesophageal cancer; Gastrointestinal, GI; Hepatobiliary cancer; Liquid biopsy; Molecular profiling; Next-generation sequencing, NGS; Pancreatic cancer; ctDNA.
© 2022. The Author(s).
Conflict of interest statement
CY, None; RM, None; RH, None; AZA, None; MK, None; ARH, Grant or research support-Merck (Merck Sharp & Dohme a subsidiary of Merck & Co, Inc.), grant or research support-Genentech, Inc., Speakers Bureau-Eisai, Speakers Bureau-BMS; BAW, Personal fees from Taiho, Lilly, Sirtex, Bayer, and Daiichi Sankyo/AstraZeneca, and grants from Ipsen outside the submitted work; JLM, Honoraria—Pfizer, Inc., and Daiichi Sankyo, Inc. Advisor—Bayer Corporation, and Taiho Oncology; MLH, None; MSN: Support from Ipsen outside the submitted work.
Figures
Similar articles
-
Clinical Applications of Circulating Tumor DNA Profiling in GI Cancers.JCO Oncol Pract. 2024 Nov;20(11):1481-1490. doi: 10.1200/OP.24.00167. Epub 2024 Nov 12. JCO Oncol Pract. 2024. PMID: 39531845 Review.
-
[Clinical Utility of Circulating Tumor DNA Analysis for Precision Medicine in Patients with Advanced Gastrointestinal Cancer].Gan To Kagaku Ryoho. 2021 Oct;48(10):1191-1196. Gan To Kagaku Ryoho. 2021. PMID: 34657046 Japanese.
-
Biomarkers for personalized medicine in GI cancers.Mol Aspects Med. 2015 Nov;45:14-27. doi: 10.1016/j.mam.2015.06.002. Epub 2015 Jun 6. Mol Aspects Med. 2015. PMID: 26054566 Review.
-
The Clinical Application of Circulating Tumor Cells and DNAs as Prognostic and Predictive Biomarkers in Gastrointestinal Cancer.Curr Cancer Drug Targets. 2021;21(8):676-688. doi: 10.2174/1568009621666210311090531. Curr Cancer Drug Targets. 2021. PMID: 33719973 Review.
-
Molecular tests and target therapies in oncology: recommendations from the Italian workshop.Future Oncol. 2021 Sep;17(26):3529-3539. doi: 10.2217/fon-2021-0286. Epub 2021 Jul 13. Future Oncol. 2021. PMID: 34254524
Cited by
-
Treatment of extended RAS/BRAF wild-type metastatic colorectal cancer with anti-EGFR antibody combinations.Pharmacogenomics. 2025 Jan-Feb;26(1-2):39-52. doi: 10.1080/14622416.2025.2479414. Epub 2025 Mar 17. Pharmacogenomics. 2025. PMID: 40097366 Review.
-
[Standardized and quality-assured predictive PD-L1 testing in the upper gastrointestinal tract. German version].Pathologie (Heidelb). 2024 Feb;45(1):51-58. doi: 10.1007/s00292-023-01215-3. Epub 2024 Jan 3. Pathologie (Heidelb). 2024. PMID: 38170268 Free PMC article. Review. German.
-
Standardized and quality-assured predictive PD-L1 testing in the upper gastrointestinal tract.J Cancer Res Clin Oncol. 2023 Nov;149(17):16231-16238. doi: 10.1007/s00432-023-05180-5. Epub 2023 Oct 24. J Cancer Res Clin Oncol. 2023. PMID: 37874352 Free PMC article. Review.
-
Gene expression in organoids: an expanding horizon.Biol Direct. 2023 Mar 25;18(1):11. doi: 10.1186/s13062-023-00360-2. Biol Direct. 2023. PMID: 36964575 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources