People With Human Immunodeficiency Virus Receiving Suppressive Antiretroviral Therapy Show Typical Antibody Durability After Dual Coronavirus Disease 2019 Vaccination and Strong Third Dose Responses
- PMID: 35668700
- PMCID: PMC9214159
- DOI: 10.1093/infdis/jiac229
People With Human Immunodeficiency Virus Receiving Suppressive Antiretroviral Therapy Show Typical Antibody Durability After Dual Coronavirus Disease 2019 Vaccination and Strong Third Dose Responses
Abstract
Background: Longer-term humoral responses to 2-dose coronavirus disease 2019 (COVID-19) vaccines remain incompletely characterized in people living with human immunodeficiency virus (HIV) (PLWH), as do initial responses to a third dose.
Methods: We measured antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein receptor-binding domain, angiotensin-converting enzyme 2 (ACE2) displacement, and viral neutralization against wild-type and Omicron strains up to 6 months after 2-dose vaccination, and 1 month after the third dose, in 99 PLWH receiving suppressive antiretroviral therapy and 152 controls.
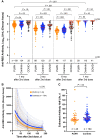
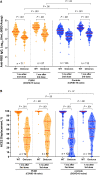
Results: Although humoral responses naturally decline after 2-dose vaccination, we found no evidence of lower antibody concentrations or faster rates of antibody decline in PLWH compared with controls after accounting for sociodemographic, health, and vaccine-related factors. We also found no evidence of poorer viral neutralization in PLWH after 2 doses, nor evidence that a low nadir CD4+ T-cell count compromised responses. Post-third-dose humoral responses substantially exceeded post-second-dose levels, though Omicron-specific responses were consistently weaker than responses against wild-type virus. Nevertheless, post-third-dose responses in PLWH were comparable to or higher than controls. An mRNA-1273 third dose was the strongest consistent correlate of higher post-third-dose responses.
Conclusion: PLWH receiving suppressive antiretroviral therapy mount strong antibody responses after 2- and 3-dose COVID-19 vaccination. Results underscore the immune benefits of third doses in light of Omicron.
Keywords: COVID-19; HIV; antibodies; humoral; immune response; neutralization; third dose; vaccines.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


Update of
-
People with HIV receiving suppressive antiretroviral therapy show typical antibody durability after dual COVID-19 vaccination, and strong third dose responses.medRxiv [Preprint]. 2022 Mar 23:2022.03.22.22272793. doi: 10.1101/2022.03.22.22272793. medRxiv. 2022. Update in: J Infect Dis. 2023 Apr 12;227(7):838-849. doi: 10.1093/infdis/jiac229. PMID: 35350205 Free PMC article. Updated. Preprint.
Comment in
-
Immune Responses to Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination in People With Human Immunodeficiency Virus: A Tale of Two Pandemics.J Infect Dis. 2023 Apr 12;227(7):835-837. doi: 10.1093/infdis/jiac231. J Infect Dis. 2023. PMID: 35668703 Free PMC article. No abstract available.
-
Comorbidity Burden and Suboptimal Immunological Responses to Coronavirus Disease 2019 Vaccination in People Living with Human Immunodeficiency Virus.J Infect Dis. 2023 Mar 1;227(5):733-735. doi: 10.1093/infdis/jiac286. J Infect Dis. 2023. PMID: 35796710 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

